Continuing Education Activity
Encapsulating peritoneal sclerosis is a complication commonly associated with patients undergoing peritoneal dialysis. If diagnosed, this disease carries high morbidity and mortality. This activity reviews the diagnosis and management of encapsulating peritoneal sclerosis and highlights the role of the interprofessional team in managing patients with this condition.
Objectives:
- Describe the pathophysiology of encapsulating peritoneal sclerosis.
- Identify risk factors for developing encapsulating peritoneal sclerosis.
- Explain the common physical exam findings associated with encapsulating peritoneal sclerosis.
- Summarize the role of the interprofessional team in providing the best outcomes for patients suffering from encapsulating peritoneal sclerosis.
Introduction
Encapsulating peritoneal sclerosis (EPS) is a clinical syndrome where a thickened fibro-collagenous peritoneal membrane can encase parts of the small intestine, leading to recurrent small bowel obstructions and malnutrition. This rare disorder is most strongly associated with long-term peritoneal dialysis, as dialysis can cause chronic inflammation and subsequent sclerosis.[1]
Multiple episodes of severe peritoneal infections or systemic inflammatory disorders can predispose a patient to EPS. EPS is also a potential late-onset complication of kidney transplantation in the absence of systemic inflammation.[2] Despite this, many cases of EPS remain idiopathic. Due to the rarity of this debilitating condition, diagnosis is often delayed.
Etiology
The etiology of encapsulating peritoneal sclerosis is not well studied. However, EPS is most commonly seen in patients on long-term peritoneal dialysis diagnosed with end-stage renal disease (ESRD). During peritoneal dialysis, the dialysate typically contains glucose or glucose degradation products which can induce inflammation in the peritoneum. This inflammation can increase endothelial permeability and fibrinogenesis, causing further damage to the peritoneum and leading to fibrin deposition.
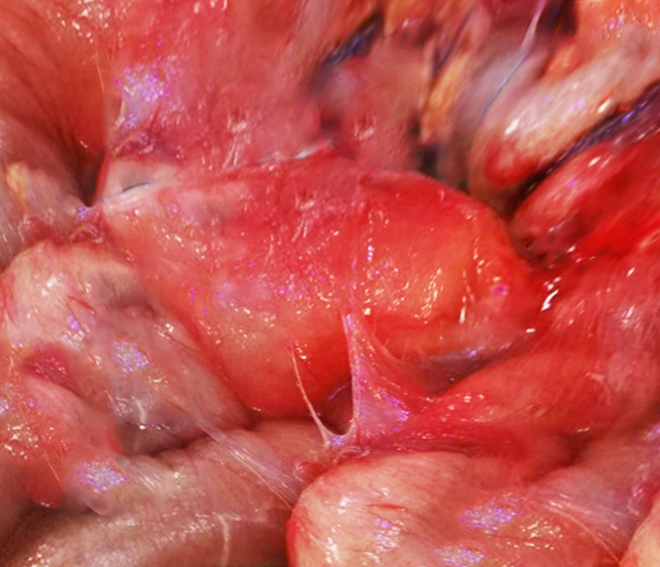
Multiple fibrin depositions can form a dense fibro-collagenous capsule around parts of the small bowel, which can cause not only acute bowel obstruction but also malnutrition. A gross image of this clinical presentation can also be seen below. Risk factors in patients on peritoneal dialysis (PD) include duration of PD, cessation of PD, renal transplantation, peritonitis, young age, low pH, high glucose within dialysate, ultrafiltration failure, and exposure to chlorhexidine.[3]
Also, there are causes of EPS that are not directly related to peritoneal dialysis. Associations include autoimmune diseases, sarcoidosis, peritoneal and intra-abdominal malignancies, chronic peritoneal ascites, intraperitoneal chemotherapy, specific dialysate fluids such as acetate or bioincompatible dialysate, abdominal surgery, endometriosis, intraperitoneal infection (particularly Staphylococcus aureus, Pseudomonas, and Mycobacterium tuberculosis and fungal species), calcineurin inhibitors, and beta-blockers administration.
Renal transplantation shows an increased risk of developing encapsulating peritoneal sclerosis, most commonly seen shortly after the transplantation. It is theorized that this could be due to the profibrotic effects and immunosuppressive medication such as calcineurin inhibitors. It could also possibly be due to the acute cessation of peritoneal dialysis.[3]
Epidemiology
The epidemiology of encapsulating peritoneal sclerosis has been studied in Scotland, Australia, New Zealand, and Japan. In contrast, there is very little data reporting the incidence of EPS in the United States. Most research available in the US has been conducted at single-center institutions. In a single center in New Haven, Connecticut, a retrospective observational cohort analysis of patients on peritoneal dialysis for five years or greater revealed the incidence of EPS as 18.4%. In this study, only 76 patients met the inclusion criteria, with 11 patients meeting the International Society for Peritoneal Dialysis criteria for the diagnosis of EPS.[4]
At another US single-center institution, a retrospective study examined a peritoneal dialysis registry, in existence for 30 years, for the incidence of EPS. In this study, 676 patients met the inclusion criteria, and the incidence of EPS was 1.2%. The study also found that the incidence of EPS rose to 15% after six years and 38% after nine years of peritoneal dialysis.[5]
Both US studies reveal not only how understudied EPS is, but the incidence of EPS increases for patients on peritoneal dialysis for at least five years or more. This finding is consistent with work in other countries like Scotland. 1238 adult patients who started peritoneal dialysis between 2000 and 2007 were identified by the Scottish Renal Registry. The incidence of EPS was 1.1% by one year, 3.4% by three years, 8.8% at four years, 9.4% at five years, and 22.2% by seven years of staying on peritoneal dialysis.[6]
In a study over 13 years of 7618 patients in Australia and New Zealand, the incidence rate was 1.8% per 1000 patients. Respective cumulative incidences of EPS were 0.3% at three years, 0.8% at five years, and 3.9% at eight years of dialysis. A Japanese study revealed the incidence (and mortality rates) for EPS were 0% (0%) at 3 years, 0.7% (0%) at five years, 2.1% (8.3%) at eight years, 5.9% (28.6%) at 10 years, 5.8% (61.5%) at 15 years, and 17.2% (100%) with 15 years or greater of peritoneal dialysis.[7]
Studies from other counties reveal the prevalence of EPS varies between 0.4 to 8.9% but can reach as high as 22.2% after seven years or more of peritoneal dialysis, as highlighted by the Scottish study.[8]
Pathophysiology
The pathophysiology of encapsulating peritoneal sclerosis is multifactorial and not fully understood. Long-term peritoneal dialysis is a significant risk factor, and patients that require treatment will experience adverse cellular changes to the peritoneal membrane due to exposure to bioincompatible peritoneal dialysis fluids. Approximately 50 to 80% of all patients on peritoneal dialysis will experience progressive sclerosis of the peritoneal membrane within one to two years of initiating treatment. The chemical composition of the solutions used in peritoneal dialysis causes oxidative stress, which damages the peritoneal membrane. The acidic pH, elevated osmolarity, and increased glucose concentration cause fibrosis, vasculopathy, and loss of peritoneal ultrafiltration.[9]
Peritoneal dialysis solutions undergo heat sterilization processes which eventually form glucose degradation products. Glucose degradation products and the increased glucose concentration are then exposed to the peritoneal membrane when the fluid is used in dialysis. Glucose degradation products then undergo another chemical reaction, forming irreversible reactive molecules known as advanced glycation end products. The advance glycation end products slowly accumulate in the peritoneal membrane after multiple rounds of dialysis that can cause peritoneal cell injury via several mechanisms. Advanced glycation end products can cause morphological changes to intracellular proteins and modify the existing structure of extracellular matrix components and receptors within peritoneal cells. Lastly, advanced glycation end products can modify proteins tightly binding to specific multi-ligand transmembrane receptors on endothelial cells and macrophages. This adverse binding can activate free radicals, pro-inflammatory cytokines, and growth factors like vascular endothelial growth factors that can lead to abnormal transcription of DNA and subsequent apoptosis. This binding can further upgrade the production of additional advanced glycation end products, potentially leading to further damage.[10]
The increased glucose-oxidation metabolism is also how glycation end products and advanced glycation end products trigger the creation of reactive oxidative species. When the fluid, high in glucose concentration, enters the peritoneal cells, it increases glucose catabolism, leading to an overproduction of electron donors to compensate. The overproduction can eventually overwhelm the cell's capacity to neutralize the reactive oxidative species, leading to a higher formation of reactive oxidative species. These reactive oxidative species lead to an increase in fibrinogenesis and endothelial permeability. Even peritoneal fluids which have a significant reduction in glucose degradation products and glucose with a neutral pH can have the slow progressive development of reactive oxidative specifies that subsequently form fibrosis that later becomes sclerotic.[11]
Peritonitis can also be a major risk factor for EPS, particularly Staphylococcus aureus, Pseudomonas, Mycobacterium tuberculosis, and fungal infections. Inflammation that occurs in the setting of peritonitis can accelerate the peritoneal transformation process and form fibrosis. One study found that peritoneal inflammation was common after dialysis catheter removal for refractory bacterial peritonitis, and these patients had a 31% likelihood of developing EPS with a mortality rate of 36%.[12]
Histopathology
Podoplanin is a type-1 transmembrane sialomucin-like glycoprotein present on the endothelium of lymphatic vessels in both submesothelial fibrotic and mesothelial cells. Podoplanin can modulate inflammatory reactions by binding to chemokines and play a potential role in the inflammatory process. One study found that patients with EPS had a diffuse infiltration of podoplanin-positive cells, which may be a suitable histopathological marker for EPS.[13]
History and Physical
The diagnosis of encapsulating peritoneal sclerosis can be made from a constellation of symptoms and the timing in which symptoms occur. EPS typically develops in a step-wise fashion with an inflammatory, encapsulating, and ileus stage. EPS can begin with an inflammatory stage in which patients can present with symptoms of fever, anemia, hypoalbuminemia, nausea, diarrhea, and an elevation in C-reactive protein. This is associated with the genesis of bowel encapsulation. The physical exam can often be unremarkable during the encapsulating phase, as the fibrotic capsule can also be too premature to inhibit intestinal peristalsis. Over time, the fibrotic capsule becomes sclerotic and causes bowel obstruction-like symptoms, which leads to the ileus stage. Early symptoms in the ileus stage are nausea, vomiting, diarrhea, intermittent abdominal pain, anorexia, and loss of appetite.
The physical exam can also be unremarkable, but some patients on peritoneal dialysis have noted blood-tinged ascites or dialysis fluid during the procedure, especially after times in which the peritoneal dialysis cavity has been dry. Late symptoms in the ileus stage can progress to severe abdominal pain, intractable vomiting, malnutrition, constipation, and weight loss. At this point in the progression of symptoms, the physical exam may reveal an abdominal mass with tenderness in light and deep palpation, hypoactive bowel sounds, and potentially abdominal rigidity. The timing in which symptoms occur is also important as most patients (70 to 90%) will be diagnosed with EPS after terminating peritoneal dialysis, which can develop as late as five years later.[14]
Evaluation
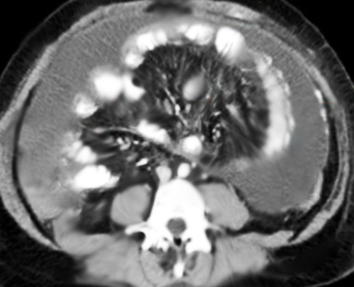
There are currently no laboratory tests for encapsulating peritoneal sclerosis, and radiographic imaging is recommended to confirm the diagnosis. CT is the definitive imaging modality of choice for EPS, with one studying affirming 100% sensitivity and 94% specificity for diagnosing EPS. CT imaging would reveal peritoneal enhancement, thickening and calcifications, bowel tethering, thickening, dilatation, and fluid septation/loculation. This can be seen in the CT imaging below. [15]
EPS can be diagnosed with clinical signs of intestinal obstruction with radiological imaging of bowel encapsulation. However, it is essential to note that other imaging modalities, such as abdominal radiography, can be used to diagnose EPS. This is because abdominal radiography does not have the same level of sensitivity or specificity. Still, air-fluid levels, signs of bowel dilation, and peritoneal calcifications can be used to suspect EPS in the setting of intestinal obstruction.
It should be noted that X-ray films can have a normal presentation despite having EPS. The most sensitive and specific method to confirm EPS can be made through a laparoscopy or laparotomy procedure, which can visualize the peritoneal thickening that encloses the bowel. But due to the invasive nature and the elevated risks of surgery, this is rarely done to diagnose EPS.[16]
Treatment / Management
Initial treatment of EPS begins with considering peritoneal dialysis cessation to halt further derangements to the peritoneal membrane. Not every patient can benefit from stopping peritoneal dialysis; thus, this decision must carefully weigh the benefits and risks, as hemodialysis would be what most patients transition to. Hemodialysis carries a different set of complications and risks when compared to peritoneal dialysis, in addition to lifestyle changes. It is also worth noting that because many cases of EPS are diagnosed years after stopping peritoneal dialysis, there is the potential for symptoms to worsen even after discontinuing.[3]
Regardless, if the decision to cease peritoneal dialysis is made, the standard of care would be switching from peritoneal dialysis to hemodialysis and removing the peritoneal dialysis catheter. To decrease the risk of abdominal adhesions, the peritoneum should be flushed twice a week with 100 to 200 mL of 1.36% dialysate with 3500 international units of heparin. Bowel rest should occur for four to twelve weeks, depending on the severity of symptoms present.[17]
Medical management of EPS includes the use of corticosteroids and/or tamoxifen. Steroids act by decreasing inflammation and the development of fibrin deposition. In treating the early stages of EPS, steroids have been proven an effective medication. Still, the effect tends to decrease in the later stages when fibrosis is significant, and bowel obstruction occurs. For these earlier stages, prednisolone dosing is 0.5 to 1.0 mg/kg/day or a pulse dose of 500 to 1000 mg of methylprednisolone for two to three days.[18]
Treatment should be continued for at least one year with a steroid taper. If using prednisolone, the taper should be 0.5 to 1.0 mg/kg daily for month one, 0.25 to 0.5 mg at 2 to 3 months, and then 10 mg at six months. CRP should be monitored, and a dramatic rise should raise the suspicion of inflammation due to bacterial peritonitis or intestinal obstruction. Tamoxifen can be an alternative treatment and may be considered the first choice considering the well-documented side effects of steroids. Despite this, tamoxifen is typically given in combination with steroids. Tamoxifen is offered in 10 to 40 mg/day doses for at least two to six months.[19]
Surgical interventions become the treatment choice when patients are at risk of obstruction due to fibrotic adhesions. A peritonectomy involves the lysis of adhesions and total ablation of sclerotic tissue in the abdomen. Surgery should only be considered in chronic patients that fail medical and conservative management and in patients with acute symptoms of bowel obstruction. Risk of surgery includes perforation of intestines, sepsis, bleeding, fistula formation, and death. There is also a risk of recurrence of adhesions and symptoms. Despite this, suturing intestine to intestine to prevent recurrence has been proposed, and considering medical management with steroids or tamoxifen might decrease risk.[20]
Nutrition and hydration should be optimized in patients with EPS. Patients who elect to treat with surgery are at high risk of refeeding syndrome, so aggressive nutritional support should be a vital aspect of the management plan for these patients in the pre & post-operative period. In research conducted in the UK, patients who underwent surgery had reportedly improved surgical outcomes when total parental nutrition was used in preoperative management care. It is important to note that TPN is not the mainstay treatment for all patients, as chronic EPS patients can benefit from close monitoring and increased oral intake.[21]
Differential Diagnosis
One important disease that can be mistaken for encapsulating peritoneal sclerosis is peritoneal encapsulation. This is also a rare disease in which a thin accessory peritoneal membrane can surround various parts of the small bowel. This forms an accessory peritoneal sac typically asymptomatic and incidentally diagnosed but can also cause small bowel obstruction symptoms. This peritoneal sac is different from EPS as peritoneal encapsulation is a congenital malformation and not one that develops from chronic inflammation.[22]
In addition, because many symptoms of EPS can be nonspecific gastrointestinal complaints, the differential diagnosis can be extensive, such as small bowel obstruction, gastroparesis, irritable bowel syndrome, pancreatic adenocarcinoma, peptic ulcer disease, pancreatitis, Crohn disease, retroperitoneal fibrosis, tumors, malignancy, hernias, and peritonitis.[23]
Prognosis
One study from Tawain reviewed three Tawainese medical centers and divided patients into a mild/moderate or severe encapsulating peritoneal sclerosis category. Severe EPS included signs and symptoms of intractable obstruction, gut ischemia, sepsis, and those that needed surgical intervention. They found that the overall mortality rate of EPS was 35% and 74% in patients in the severe EPS group.[24] Encapsulating peritoneal sclerosis carries high morbidity and a mortality rate that can approach as high as 50% within 12 months of diagnosis.[25]
Complications
Complications can arise from both medical treatment and surgical intervention. Tamoxifen has been associated with a higher risk of endometrial adenocarcinoma, uterine sarcoma, pulmonary embolism, and stroke in female patients.[26] Prednisone has reported side effects of tertiary adrenal insufficiency, hypertension, sodium retention, decreased serum potassium, fluid retention, psychiatric disturbances, cushingoid features, and hyperglycemia. From surgical intervention, complications include excessive bleeding, inadvertent bowel injury, abscess formation, surgical site infection, perforation of intestinal contents, deep vein thrombosis, atelectasis, and sepsis.[27]
Many of these complications occur in the postoperative period. Thus prophylactic management with DVT prophylaxis, antibiotics during preop, insertion/removal of foley catheters, incentive spirometry, and slowly escalating the diet as tolerated should be considered.[28]
Deterrence and Patient Education
Patient education is imperative in improving a patient's health literacy. By helping the patient understand the condition, the risks/benefits of treatment, and complications, they can make more informed decisions regarding their care. In addition, physicians and the healthcare team can implement the teach-back method, avoiding medical jargon, actively engaging in patient questions, and explaining the diagnosis to achieve this. Thus it is prudent to inform any patient considering peritoneal dialysis of the benefits/risk, especially concerning an increased risk in encapsulating peritoneal dialysis.
By effectively educating a patient on this complication from dialysis, a patient will be more aware of the signs and symptoms to look out for to catch EPS earlier. Patient education could decrease the risk of delayed diagnosis and ensure proper treatment. This method applies not only to the disease in question but to management options as well. There are numerous risks/benefits to using steroids, tamoxifen, and surgical intervention. A better understanding of these interventions can ensure a more informed decision is made.[29]
Enhancing Healthcare Team Outcomes
Encapsulating peritoneal sclerosis requires a multi-faceted approach regarding proper diagnosis, treatment, and follow-up care. Adequate communication and trust are pivotal because various clinicians, surgeons, mid-level providers, nurses, pharmacists, and technicians are involved in patient education. As highlighted above, the healthcare team can implement the teach-back method, avoid medical jargon, actively engage in patients' questions, and explain the diagnosis. It can help patients make more informed decisions regarding their care. Proper communication ensures that the healthcare team efficiently and effectively provides patients with the highest quality of care. It is essential to recognize the complex interplays of various healthcare providers in the treatment of encapsulating peritoneal sclerosis.
Firstly, EPS patients are typically on peritoneal dialysis, typically under the care of a nephrologist managed by a dialysis-trained healthcare team. This team can consist of mid-level providers, nurses, medical assistants, and dialysis-trained technicians who actively manage patients' dialysis needs. In addition, should a patient become hospitalized for symptoms pertaining to suspected EPS, consider the radiologist that reads the CT scan, the pharmacist that manages the tamoxifen or steroids, the primary care team which coordinates care with surgery, the surgeon that proceeds with lysis of adhesions and the numerous other healthcare providers. This dynamic interplay necessitates the vital role of good communication in ensuring effective care, as one healthcare provider's actions can affect another healthcare provider's interpretation and management plans.[30]
Regarding the decision to proceed with emergent surgical intervention highlighting a patient's objective deterioration, understanding a patient's wishes, and involving the patient's family in the decision can help enhance care. In addition, trust is pivotal in this example, and all other healthcare interactions highlighted above. By trusting what another clinician writes regarding a patient's history and correlating it with the pertinent laboratory/imaging/physical exam findings made possible by the healthcare team, the team becomes more efficient. And by working efficiently and effectively on behalf of a patient, trust can develop. A meta-analysis of four databases on the trust between healthcare professionals and health outcomes revealed that patients reported more beneficial health behaviors, fewer symptoms, higher quality of life, and more satisfaction with treatment when they had higher trust in their healthcare professional.[31] [Level 1]