[1]
Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG : an international journal of obstetrics and gynaecology. 2017 Sep:124(10):1501-1512. doi: 10.1111/1471-0528.14640. Epub 2017 May 13
[PubMed PMID: 28296146]
Level 1 (high-level) evidence
[2]
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Gynecology., Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstetrics and gynecology. 2021 Jun 1
[PubMed PMID: 34011888]
[3]
Giuliani E,As-Sanie S,Marsh EE, Epidemiology and management of uterine fibroids. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2020 Apr
[PubMed PMID: 31960950]
[4]
Wallach EE,Vlahos NF, Uterine myomas: an overview of development, clinical features, and management. Obstetrics and gynecology. 2004 Aug;
[PubMed PMID: 15292018]
Level 3 (low-level) evidence
[5]
Guo XC,Segars JH, The impact and management of fibroids for fertility: an evidence-based approach. Obstetrics and gynecology clinics of North America. 2012 Dec
[PubMed PMID: 23182558]
[6]
Buttram VC Jr,Reiter RC, Uterine leiomyomata: etiology, symptomatology, and management. Fertility and sterility. 1981 Oct
[PubMed PMID: 7026295]
[7]
Wise LA,Palmer JR,Harlow BL,Spiegelman D,Stewart EA,Adams-Campbell LL,Rosenberg L, Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. American journal of epidemiology. 2004 Jan 15
[PubMed PMID: 14718211]
[8]
Pavone D,Clemenza S,Sorbi F,Fambrini M,Petraglia F, Epidemiology and Risk Factors of Uterine Fibroids. Best practice & research. Clinical obstetrics & gynaecology. 2018 Jan
[PubMed PMID: 29054502]
[9]
Takeda T,Sakata M,Isobe A,Miyake A,Nishimoto F,Ota Y,Kamiura S,Kimura T, Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecologic and obstetric investigation. 2008
[PubMed PMID: 18230910]
Level 2 (mid-level) evidence
[10]
Ross RK,Pike MC,Vessey MP,Bull D,Yeates D,Casagrande JT, Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. British medical journal (Clinical research ed.). 1986 Aug 9
[PubMed PMID: 3730804]
[11]
Flake GP,Andersen J,Dixon D, Etiology and pathogenesis of uterine leiomyomas: a review. Environmental health perspectives. 2003 Jun
[PubMed PMID: 12826476]
Level 3 (low-level) evidence
[13]
Ishikawa H,Ishi K,Serna VA,Kakazu R,Bulun SE,Kurita T, Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010 Jun
[PubMed PMID: 20375184]
[14]
Bertsch E,Qiang W,Zhang Q,Espona-Fiedler M,Druschitz S,Liu Y,Mittal K,Kong B,Kurita T,Wei JJ, MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 Aug
[PubMed PMID: 24390224]
Level 2 (mid-level) evidence
[16]
Brandon DD,Erickson TE,Keenan EJ,Strawn EY,Novy MJ,Burry KA,Warner C,Clinton GM, Estrogen receptor gene expression in human uterine leiomyomata. The Journal of clinical endocrinology and metabolism. 1995 Jun
[PubMed PMID: 7775635]
[17]
Islam MS,Ciavattini A,Petraglia F,Castellucci M,Ciarmela P, Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Human reproduction update. 2018 Jan 1;
[PubMed PMID: 29186429]
[18]
Stewart EA,Friedman AJ,Peck K,Nowak RA, Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. The Journal of clinical endocrinology and metabolism. 1994 Sep
[PubMed PMID: 8077380]
[20]
Nishino M,Togashi K,Nakai A,Hayakawa K,Kanao S,Iwasaku K,Fujii S, Uterine contractions evaluated on cine MR imaging in patients with uterine leiomyomas. European journal of radiology. 2005 Jan
[PubMed PMID: 15607866]
[21]
Borah BJ,Nicholson WK,Bradley L,Stewart EA, The impact of uterine leiomyomas: a national survey of affected women. American journal of obstetrics and gynecology. 2013 Oct;
[PubMed PMID: 23891629]
Level 3 (low-level) evidence
[22]
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons., Myomas and reproductive function. Fertility and sterility. 2008 Nov
[PubMed PMID: 19007608]
[23]
Cook H,Ezzati M,Segars JH,McCarthy K, The impact of uterine leiomyomas on reproductive outcomes. Minerva ginecologica. 2010 Jun;
[PubMed PMID: 20595947]
[24]
Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011 Apr:113(1):3-13. doi: 10.1016/j.ijgo.2010.11.011. Epub 2011 Feb 22
[PubMed PMID: 21345435]
[25]
Munro MG,Critchley HOD,Fraser IS,FIGO Menstrual Disorders Committee., The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2018 Dec;
[PubMed PMID: 30198563]
[26]
Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstetrics and gynecology. 2012 Jul:120(1):197-206. doi: 10.1097/AOG.0b013e318262e320. Epub
[PubMed PMID: 22914421]
[27]
Dueholm M,Lundorf E,Hansen ES,Ledertoug S,Olesen F, Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. American journal of obstetrics and gynecology. 2002 Mar;
[PubMed PMID: 11904599]
[28]
Woźniak A,Woźniak S, Ultrasonography of uterine leiomyomas. Przeglad menopauzalny = Menopause review. 2017 Dec;
[PubMed PMID: 29483851]
[29]
Cicinelli E,Romano F,Anastasio PS,Blasi N,Parisi C,Galantino P, Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstetrics and gynecology. 1995 Jan
[PubMed PMID: 7800322]
[30]
Omary RA,Vasireddy S,Chrisman HB,Ryu RK,Pereles FS,Carr JC,Resnick SA,Nemcek AA Jr,Vogelzang RL, The effect of pelvic MR imaging on the diagnosis and treatment of women with presumed symptomatic uterine fibroids. Journal of vascular and interventional radiology : JVIR. 2002 Nov;
[PubMed PMID: 12427815]
[31]
Sohn GS,Cho S,Kim YM,Cho CH,Kim MR,Lee SR,Working Group of Society of Uterine Leiomyoma., Current medical treatment of uterine fibroids. Obstetrics & gynecology science. 2018 Mar
[PubMed PMID: 29564309]
[32]
Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. The Cochrane database of systematic reviews. 2019 Sep 19:9(9):CD000400. doi: 10.1002/14651858.CD000400.pub4. Epub 2019 Sep 19
[PubMed PMID: 31535715]
Level 1 (high-level) evidence
[33]
Lethaby A,Wise MR,Weterings MA,Bofill Rodriguez M,Brown J, Combined hormonal contraceptives for heavy menstrual bleeding. The Cochrane database of systematic reviews. 2019 Feb 11;
[PubMed PMID: 30742315]
Level 1 (high-level) evidence
[34]
Sayed GH,Zakherah MS,El-Nashar SA,Shaaban MM, A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011 Feb
[PubMed PMID: 21092958]
Level 1 (high-level) evidence
[35]
Bahamondes L, Fernandes A, Monteiro I, Bahamondes MV. Long-acting reversible contraceptive (LARCs) methods. Best practice & research. Clinical obstetrics & gynaecology. 2020 Jul:66():28-40. doi: 10.1016/j.bpobgyn.2019.12.002. Epub 2019 Dec 20
[PubMed PMID: 32014434]
[36]
Kokolina VF,Iakunina LN,Dubovaia TN, [The system of regulation of blood coagulation in girls with juvenile uterine hemorrhage]. Pediatriia. 1990
[PubMed PMID: 2399071]
[37]
Wu JP,Pickle S, Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014 Jun
[PubMed PMID: 24679478]
[38]
Sergison JE,Maldonado LY,Gao X,Hubacher D, Levonorgestrel intrauterine system associated amenorrhea: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2019 May
[PubMed PMID: 30527945]
Level 1 (high-level) evidence
[39]
Jiang W,Shen Q,Chen M,Wang Y,Zhou Q,Zhu X,Zhu X, Levonorgestrel-releasing intrauterine system use in premenopausal women with symptomatic uterine leiomyoma: a systematic review. Steroids. 2014 Aug;
[PubMed PMID: 24832215]
Level 1 (high-level) evidence
[40]
Newton CL,Riekert C,Millar RP, Gonadotropin-releasing hormone analog therapeutics. Minerva ginecologica. 2018 Oct
[PubMed PMID: 30264955]
[41]
Lethaby A,Puscasiu L,Vollenhoven B, Preoperative medical therapy before surgery for uterine fibroids. The Cochrane database of systematic reviews. 2017 Nov 15
[PubMed PMID: 29139105]
Level 1 (high-level) evidence
[42]
Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. American journal of obstetrics and gynecology. 2016 Jan:214(1):31-44. doi: 10.1016/j.ajog.2015.07.044. Epub 2015 Aug 5
[PubMed PMID: 26254516]
[43]
Schlaff WD,Ackerman RT,Al-Hendy A,Archer DF,Barnhart KT,Bradley LD,Carr BR,Feinberg EC,Hurtado SM,Kim J,Liu R,Mabey RG Jr,Owens CD,Poindexter A,Puscheck EE,Rodriguez-Ginorio H,Simon JA,Soliman AM,Stewart EA,Watts NB,Muneyyirci-Delale O, Elagolix for Heavy Menstrual Bleeding in Women with Uterine Fibroids. The New England journal of medicine. 2020 Jan 23
[PubMed PMID: 31971678]
[44]
Simon JA,Al-Hendy A,Archer DF,Barnhart KT,Bradley LD,Carr BR,Dayspring T,Feinberg EC,Gillispie V,Hurtado S,Kim J,Liu R,Owens CD,Muneyyirci-Delale O,Wang A,Watts NB,Schlaff WD, Elagolix Treatment for Up to 12 Months in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas. Obstetrics and gynecology. 2020 Jun
[PubMed PMID: 32459423]
[45]
Al-Hendy A,Lukes AS,Poindexter AN 3rd,Venturella R,Villarroel C,Critchley HOD,Li Y,McKain L,Arjona Ferreira JC,Langenberg AGM,Wagman RB,Stewart EA, Treatment of Uterine Fibroid Symptoms with Relugolix Combination Therapy. The New England journal of medicine. 2021 Feb 18
[PubMed PMID: 33596357]
[46]
Ali M,Al-Hendy A, Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biology of reproduction. 2017 Sep 1
[PubMed PMID: 29025038]
[47]
Murji A,Whitaker L,Chow TL,Sobel ML, Selective progesterone receptor modulators (SPRMs) for uterine fibroids. The Cochrane database of systematic reviews. 2017 Apr 26
[PubMed PMID: 28444736]
Level 1 (high-level) evidence
[48]
Donnez J,Tatarchuk TF,Bouchard P,Puscasiu L,Zakharenko NF,Ivanova T,Ugocsai G,Mara M,Jilla MP,Bestel E,Terrill P,Osterloh I,Loumaye E,PEARL I Study Group., Ulipristal acetate versus placebo for fibroid treatment before surgery. The New England journal of medicine. 2012 Feb 2
[PubMed PMID: 22296075]
[49]
Donnez J,Vázquez F,Tomaszewski J,Nouri K,Bouchard P,Fauser BC,Barlow DH,Palacios S,Donnez O,Bestel E,Osterloh I,Loumaye E,PEARL III and PEARL III Extension Study Group., Long-term treatment of uterine fibroids with ulipristal acetate ☆. Fertility and sterility. 2014 Jun;
[PubMed PMID: 24630081]
[50]
Gurates B,Parmaksiz C,Kilic G,Celik H,Kumru S,Simsek M, Treatment of symptomatic uterine leiomyoma with letrozole. Reproductive biomedicine online. 2008 Oct
[PubMed PMID: 18854113]
[51]
Bulun SE,Imir G,Utsunomiya H,Thung S,Gurates B,Tamura M,Lin Z, Aromatase in endometriosis and uterine leiomyomata. The Journal of steroid biochemistry and molecular biology. 2005 May
[PubMed PMID: 16024248]
[52]
Song H,Lu D,Navaratnam K,Shi G, Aromatase inhibitors for uterine fibroids. The Cochrane database of systematic reviews. 2013 Oct 23
[PubMed PMID: 24151065]
Level 1 (high-level) evidence
[53]
Leone Roberti Maggiore U,Scala C,Venturini PL,Ferrero S, Preoperative treatment with letrozole in patients undergoing laparoscopic myomectomy of large uterine myomas: a prospective non-randomized study. European journal of obstetrics, gynecology, and reproductive biology. 2014 Oct
[PubMed PMID: 25150954]
Level 2 (mid-level) evidence
[54]
Wamsteker K,Emanuel MH,de Kruif JH, Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstetrics and gynecology. 1993 Nov;
[PubMed PMID: 8414318]
[55]
Emanuel MH,Hart A,Wamsteker K,Lammes F, An analysis of fluid loss during transcervical resection of submucous myomas. Fertility and sterility. 1997 Nov;
[PubMed PMID: 9389820]
[56]
Hart R,Molnár BG,Magos A, Long term follow up of hysteroscopic myomectomy assessed by survival analysis. British journal of obstetrics and gynaecology. 1999 Jul
[PubMed PMID: 10428527]
[57]
Van Dongen H,Emanuel MH,Smeets MJ,Trimbos B,Jansen FW, Follow-up after incomplete hysteroscopic removal of uterine fibroids. Acta obstetricia et gynecologica Scandinavica. 2006
[PubMed PMID: 17260223]
[58]
Sandberg EM,Tummers FHMP,Cohen SL,van den Haak L,Dekkers OM,Jansen FW, Reintervention risk and quality of life outcomes after uterine-sparing interventions for fibroids: a systematic review and meta-analysis. Fertility and sterility. 2018 Apr
[PubMed PMID: 29653718]
Level 2 (mid-level) evidence
[59]
Polena V,Mergui JL,Perrot N,Poncelet C,Barranger E,Uzan S, Long-term results of hysteroscopic myomectomy in 235 patients. European journal of obstetrics, gynecology, and reproductive biology. 2007 Feb
[PubMed PMID: 16530319]
[60]
Laughlin-Tommaso SK,Lu D,Thomas L,Diamond MP,Wallace K,Wegienka G,Vines AI,Anchan RM,Wang T,Maxwell GL,Jacoby V,Marsh EE,Spies JB,Nicholson WK,Stewart EA,Myers ER, Short-term quality of life after myomectomy for uterine fibroids from the COMPARE-UF Fibroid Registry. American journal of obstetrics and gynecology. 2020 Apr
[PubMed PMID: 31678093]
Level 2 (mid-level) evidence
[61]
Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertility and sterility. 2009 Apr:91(4):1215-23. doi: 10.1016/j.fertnstert.2008.01.051. Epub 2008 Mar 12
[PubMed PMID: 18339376]
Level 1 (high-level) evidence
[62]
Vercellini P,Zàina B,Yaylayan L,Pisacreta A,De Giorgi O,Crosignani PG, Hysteroscopic myomectomy: long-term effects on menstrual pattern and fertility. Obstetrics and gynecology. 1999 Sep;
[PubMed PMID: 10472856]
[63]
Jansen FW,Vredevoogd CB,van Ulzen K,Hermans J,Trimbos JB,Trimbos-Kemper TC, Complications of hysteroscopy: a prospective, multicenter study. Obstetrics and gynecology. 2000 Aug
[PubMed PMID: 10908775]
Level 2 (mid-level) evidence
[64]
The Use of Hysteroscopy for the Diagnosis and Treatment of Intrauterine Pathology: ACOG Committee Opinion, Number 800. Obstetrics and gynecology. 2020 Mar
[PubMed PMID: 32080054]
Level 3 (low-level) evidence
[65]
Broder MS,Goodwin S,Chen G,Tang LJ,Costantino MM,Nguyen MH,Yegul TN,Erberich H, Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstetrics and gynecology. 2002 Nov
[PubMed PMID: 12423842]
[66]
Bhave Chittawar P,Franik S,Pouwer AW,Farquhar C, Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. The Cochrane database of systematic reviews. 2014 Oct 21
[PubMed PMID: 25331441]
Level 1 (high-level) evidence
[67]
Sizzi O,Rossetti A,Malzoni M,Minelli L,La Grotta F,Soranna L,Panunzi S,Spagnolo R,Imperato F,Landi S,Fiaccamento A,Stola E, Italian multicenter study on complications of laparoscopic myomectomy. Journal of minimally invasive gynecology. 2007 Jul-Aug
[PubMed PMID: 17630163]
Level 2 (mid-level) evidence
[68]
Sandberg EM,Cohen SL,Jansen FW,Einarsson JI, Analysis of Risk Factors for Intraoperative Conversion of Laparoscopic Myomectomy. Journal of minimally invasive gynecology. 2016 Mar-Apr
[PubMed PMID: 26546180]
[69]
Dubuisson JB,Fauconnier A,Fourchotte V,Babaki-Fard K,Coste J,Chapron C, Laparoscopic myomectomy: predicting the risk of conversion to an open procedure. Human reproduction (Oxford, England). 2001 Aug
[PubMed PMID: 11473973]
[70]
Dubuisson JB,Fauconnier A,Chapron C,Kreiker G,Nörgaard C, Second look after laparoscopic myomectomy. Human reproduction (Oxford, England). 1998 Aug;
[PubMed PMID: 9756277]
[71]
Mais V,Ajossa S,Piras B,Guerriero S,Marongiu D,Melis GB, Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Human reproduction (Oxford, England). 1995 Dec
[PubMed PMID: 8822429]
Level 1 (high-level) evidence
[72]
Hasson HM,Rotman C,Rana N,Sistos F,Dmowski WP, Laparoscopic myomectomy. Obstetrics and gynecology. 1992 Nov
[PubMed PMID: 1407934]
[73]
Vargas MV,Moawad GN,Sievers C,Opoku-Anane J,Marfori CQ,Tyan P,Robinson JK, Feasibility, Safety, and Prediction of Complications for Minimally Invasive Myomectomy in Women With Large and Numerous Myomata. Journal of minimally invasive gynecology. 2017 Feb
[PubMed PMID: 27939896]
Level 2 (mid-level) evidence
[74]
Gkegkes ID,Iatrakis G,Iavazzo PE,Bakalianou K,Iavazzo C, Robotic Management of Fibroids: Discussion of Use, Criteria and Advantages. Acta medica (Hradec Kralove). 2020
[PubMed PMID: 32771070]
[75]
Iavazzo C,Mamais I,Gkegkes ID, Robotic assisted vs laparoscopic and/or open myomectomy: systematic review and meta-analysis of the clinical evidence. Archives of gynecology and obstetrics. 2016 Jul;
[PubMed PMID: 26969650]
Level 1 (high-level) evidence
[76]
Gingold JA,Gueye NA,Falcone T, Minimally Invasive Approaches to Myoma Management. Journal of minimally invasive gynecology. 2018 Feb
[PubMed PMID: 28734973]
[77]
Metwally M,Cheong YC,Horne AW, Surgical treatment of fibroids for subfertility. The Cochrane database of systematic reviews. 2012 Nov 14
[PubMed PMID: 23152222]
Level 1 (high-level) evidence
[78]
Barakat EE,Bedaiwy MA,Zimberg S,Nutter B,Nosseir M,Falcone T, Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstetrics and gynecology. 2011 Feb
[PubMed PMID: 21252737]
[79]
Kotani Y,Tobiume T,Fujishima R,Shigeta M,Takaya H,Nakai H,Suzuki A,Tsuji I,Mandai M,Matsumura N, Recurrence of uterine myoma after myomectomy: Open myomectomy versus laparoscopic myomectomy. The journal of obstetrics and gynaecology research. 2018 Feb;
[PubMed PMID: 29227004]
[80]
Chen R,Su Z,Yang L,Xin L,Yuan X,Wang Y, The effects and costs of laparoscopic versus abdominal myomectomy in patients with uterine fibroids: a systematic review and meta-analysis. BMC surgery. 2020 Mar 20;
[PubMed PMID: 32192462]
Level 1 (high-level) evidence
[81]
Practice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org.,Practice Committee of the American Society for Reproductive Medicine., Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertility and sterility. 2017 Sep
[PubMed PMID: 28865538]
[82]
Desai P,Patel P, Fibroids, infertility and laparoscopic myomectomy. Journal of gynecological endoscopy and surgery. 2011 Jan
[PubMed PMID: 22442534]
Level 2 (mid-level) evidence
[83]
Casini ML,Rossi F,Agostini R,Unfer V, Effects of the position of fibroids on fertility. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2006 Feb
[PubMed PMID: 16603437]
Level 2 (mid-level) evidence
[84]
Hart R,Khalaf Y,Yeong CT,Seed P,Taylor A,Braude P, A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Human reproduction (Oxford, England). 2001 Nov;
[PubMed PMID: 11679530]
[85]
Bulletti C,De Ziegler D,Polli V,Flamigni C, The role of leiomyomas in infertility. The Journal of the American Association of Gynecologic Laparoscopists. 1999 Nov;
[PubMed PMID: 10548702]
[86]
de Bruijn AM, Ankum WM, Reekers JA, Birnie E, van der Kooij SM, Volkers NA, Hehenkamp WJ. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. American journal of obstetrics and gynecology. 2016 Dec:215(6):745.e1-745.e12. doi: 10.1016/j.ajog.2016.06.051. Epub 2016 Jul 5
[PubMed PMID: 27393268]
Level 1 (high-level) evidence
[87]
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. The Cochrane database of systematic reviews. 2014 Dec 26:(12):CD005073. doi: 10.1002/14651858.CD005073.pub4. Epub 2014 Dec 26
[PubMed PMID: 25541260]
Level 1 (high-level) evidence
[88]
Mara M,Maskova J,Fucikova Z,Kuzel D,Belsan T,Sosna O, Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovascular and interventional radiology. 2008 Jan-Feb
[PubMed PMID: 17943348]
Level 1 (high-level) evidence
[89]
Hartmann KE,Fonnesbeck C,Surawicz T,Krishnaswami S,Andrews JC,Wilson JE,Velez-Edwards D,Kugley S,Sathe NA, Management of Uterine Fibroids. 2017 Dec
[PubMed PMID: 30789683]
[90]
van der Kooij SM,Hehenkamp WJ,Volkers NA,Birnie E,Ankum WM,Reekers JA, Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 5-year outcome from the randomized EMMY trial. American journal of obstetrics and gynecology. 2010 Aug
[PubMed PMID: 20579960]
Level 1 (high-level) evidence
[91]
Carrillo TC, Uterine Artery Embolization in the Management of Symptomatic Uterine Fibroids: An Overview of Complications and Follow-up. Seminars in interventional radiology. 2008 Dec
[PubMed PMID: 21326579]
Level 3 (low-level) evidence
[92]
Homer H,Saridogan E, Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertility and sterility. 2010 Jun
[PubMed PMID: 19361799]
[93]
Hesley GK,Gorny KR,Woodrum DA, MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovascular and interventional radiology. 2013 Feb
[PubMed PMID: 22453202]
[94]
Funaki K,Fukunishi H,Funaki T,Sawada K,Kaji Y,Maruo T, Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. American journal of obstetrics and gynecology. 2007 Feb
[PubMed PMID: 17306674]
[95]
Funaki K,Fukunishi H,Sawada K, Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009 Nov
[PubMed PMID: 19852041]
Level 2 (mid-level) evidence
[96]
Gorny KR,Woodrum DA,Brown DL,Henrichsen TL,Weaver AL,Amrami KK,Hangiandreou NJ,Edmonson HA,Bouwsma EV,Stewart EA,Gostout BS,Ehman DA,Hesley GK, Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. Journal of vascular and interventional radiology : JVIR. 2011 Jun
[PubMed PMID: 21482137]
[97]
Hesley GK,Felmlee JP,Gebhart JB,Dunagan KT,Gorny KR,Kesler JB,Brandt KR,Glantz JN,Gostout BS, Noninvasive treatment of uterine fibroids: early Mayo Clinic experience with magnetic resonance imaging-guided focused ultrasound. Mayo Clinic proceedings. 2006 Jul
[PubMed PMID: 16835973]
[98]
Clark NA,Mumford SL,Segars JH, Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Current opinion in obstetrics & gynecology. 2014 Jun
[PubMed PMID: 24751998]
Level 3 (low-level) evidence
[99]
Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, Hesley G, Kim HS, Hengst S, Gedroyc WM. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertility and sterility. 2006 Jan:85(1):22-9
[PubMed PMID: 16412721]
Level 2 (mid-level) evidence
[100]
Lee BB,Yu SP, Radiofrequency Ablation of Uterine Fibroids: a Review. Current obstetrics and gynecology reports. 2016;
[PubMed PMID: 27917310]
[101]
Chudnoff SG,Berman JM,Levine DJ,Harris M,Guido RS,Banks E, Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstetrics and gynecology. 2013 May;
[PubMed PMID: 23635746]
[102]
Garza Leal JG,Hernandez Leon I,Castillo Saenz L,Lee BB, Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 Ablation System. Journal of minimally invasive gynecology. 2011 May-Jun;
[PubMed PMID: 21545960]
Level 2 (mid-level) evidence
[103]
Berman JM,Shashoua A,Olson C,Brucker S,Thiel JA,Bhagavath B, Case Series of Reproductive Outcomes after Laparoscopic Radiofrequency Ablation of Symptomatic Myomas. Journal of minimally invasive gynecology. 2020 Mar - Apr;
[PubMed PMID: 31238151]
Level 2 (mid-level) evidence
[104]
Bradley LD,Pasic RP,Miller LE, Clinical Performance of Radiofrequency Ablation for Treatment of Uterine Fibroids: Systematic Review and Meta-Analysis of Prospective Studies. Journal of laparoendoscopic
[PubMed PMID: 31702440]
Level 1 (high-level) evidence
[105]
Berman JM,Guido RS,Garza Leal JG,Pemueller RR,Whaley FS,Chudnoff SG,Halt Study Group., Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. Journal of minimally invasive gynecology. 2014 Sep-Oct;
[PubMed PMID: 24613404]
[106]
Kepkep K,Tuncay YA,Göynümer G,Tutal E, Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound in obstetrics
[PubMed PMID: 17659649]
[107]
Atri M,Reinhold C,Mehio AR,Chapman WB,Bret PM, Adenomyosis: US features with histologic correlation in an in-vitro study. Radiology. 2000 Jun;
[PubMed PMID: 10831700]
[108]
Van den Bosch T,Van Schoubroeck D, Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best practice
[PubMed PMID: 29506961]
[110]
Dave KS,Chauhan A,Bhansali R,Arora R,Purohit S, Uterine carcinosarcomas: 8-year single center experience of 25 cases. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical
[PubMed PMID: 22557781]
Level 3 (low-level) evidence
[111]
Faria SC,Devine CE,Rao B,Sagebiel T,Bhosale P, Imaging and Staging of Endometrial Cancer. Seminars in ultrasound, CT, and MR. 2019 Aug;
[PubMed PMID: 31375169]
[112]
Yoo EH,Lee PI,Huh CY,Kim DH,Lee BS,Lee JK,Kim D, Predictors of leiomyoma recurrence after laparoscopic myomectomy. Journal of minimally invasive gynecology. 2007 Nov-Dec;
[PubMed PMID: 17980328]
[113]
Odejinmi F,Strong S,Sideris M,Mallick R, Caesarean section in women following an abdominal myomectomy: a choice or a need? Facts, views
[PubMed PMID: 32696025]

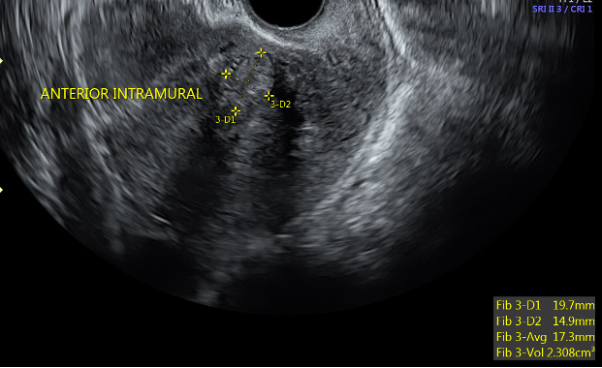
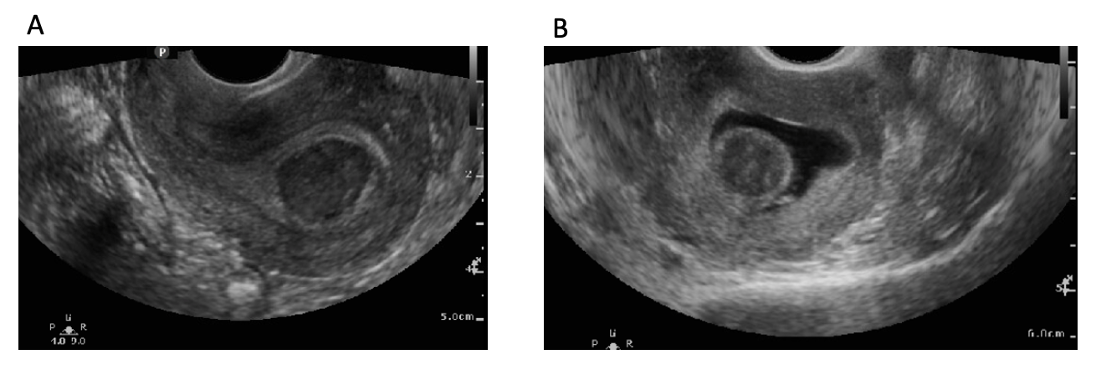
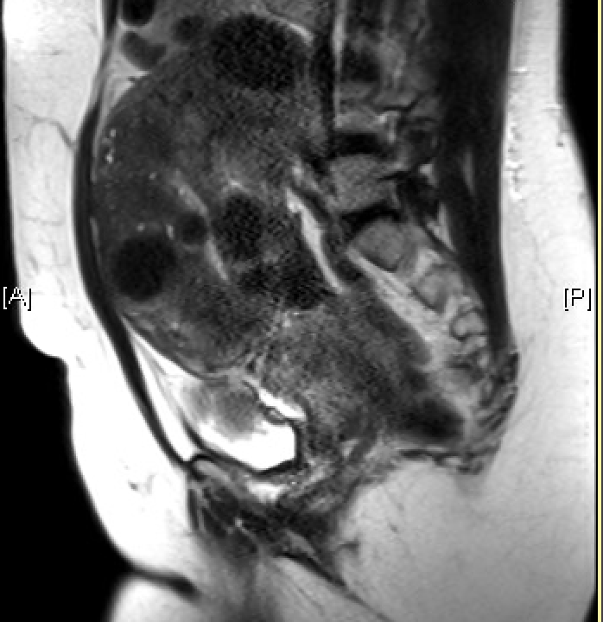
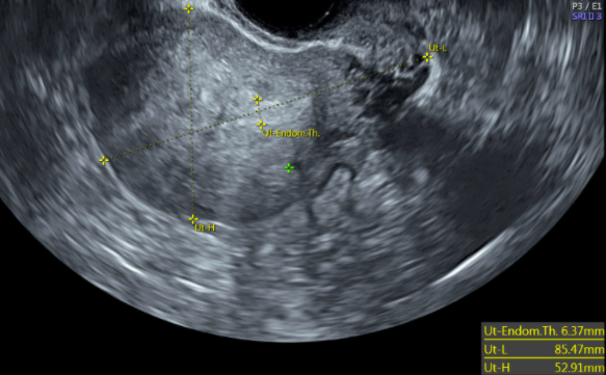
![FIGO Leiomyoma Subclassification System, 2018 [30609040]](/pictures/getimagecontent//18370)