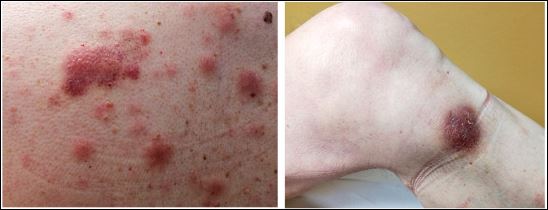
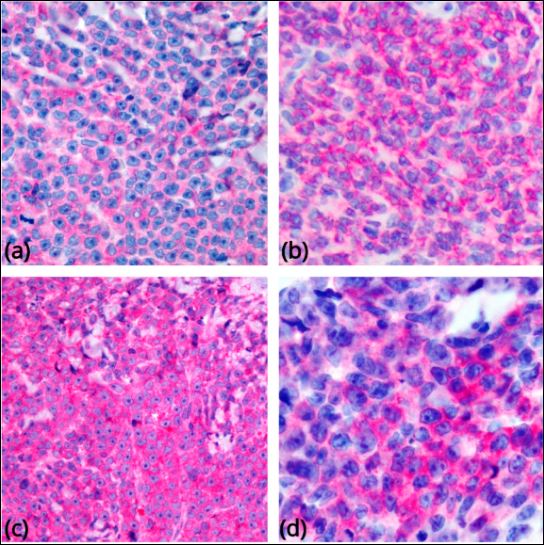
[1]
Brody JP, Allen S, Schulman P, Sun T, Chan WC, Friedman HD, Teichberg S, Koduru P, Cone RW, Loughran TP Jr. Acute agranular CD4-positive natural killer cell leukemia. Comprehensive clinicopathologic studies including virologic and in vitro culture with inducing agents. Cancer. 1995 May 15:75(10):2474-83
[PubMed PMID: 7736391]
[2]
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19:127(20):2391-405. doi: 10.1182/blood-2016-03-643544. Epub 2016 Apr 11
[PubMed PMID: 27069254]
[3]
Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, Lunghi M, Pica G, Onida F, Cattaneo C, Piccaluga PP, Di Bona E, Todisco E, Musto P, Spadea A, D'Arco A, Pileri S, Leone G, Amadori S, Facchetti F, GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute Leukemia Working Party). Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013 Feb:98(2):239-46. doi: 10.3324/haematol.2012.072645. Epub 2012 Oct 12
[PubMed PMID: 23065521]
[4]
Feuillard J, Jacob MC, Valensi F, Maynadié M, Gressin R, Chaperot L, Arnoulet C, Brignole-Baudouin F, Drénou B, Duchayne E, Falkenrodt A, Garand R, Homolle E, Husson B, Kuhlein E, Le Calvez G, Sainty D, Sotto MF, Trimoreau F, Béné MC. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002 Mar 1:99(5):1556-63
[PubMed PMID: 11861268]
[5]
Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, Dereure O, Wetterwald M, d'Incan M, Grange F, Cornillon J, Tertian G, Maubec E, Saiag P, Barete S, Templier I, Aubin F, Dalle S. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. The British journal of dermatology. 2013 Sep:169(3):579-86. doi: 10.1111/bjd.12412. Epub
[PubMed PMID: 23646868]
[6]
Reizis B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity. 2019 Jan 15:50(1):37-50. doi: 10.1016/j.immuni.2018.12.027. Epub
[PubMed PMID: 30650380]
[7]
Nomburg J, Bullman S, Chung SS, Togami K, Walker MA, Griffin GK, Morgan EA, LeBoeuf NR, DeCaprio JA, Meyerson M, Lane AA. Comprehensive metagenomic analysis of blastic plasmacytoid dendritic cell neoplasm. Blood advances. 2020 Mar 24:4(6):1006-1011. doi: 10.1182/bloodadvances.2019001260. Epub
[PubMed PMID: 32182365]
Level 3 (low-level) evidence
[8]
Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, Meijer CJ, Courville P, Joly P, Grange F, De Muret A, Machet L, Dompmartin A, Bosq J, Durlach A, Bernard P, Dalac S, Dechelotte P, D'Incan M, Wechsler J, Teitell MA. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. American journal of clinical pathology. 2005 May:123(5):662-75
[PubMed PMID: 15981806]
[9]
Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. The Journal of experimental medicine. 1997 Mar 17:185(6):1101-11
[PubMed PMID: 9091583]
[10]
Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nature immunology. 2000 Oct:1(4):305-10
[PubMed PMID: 11017101]
[11]
Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA, Pileri A Jr, Mancini M, Rossi M, Ricci F, Gazzola A, Melle F, Mannu C, Ulbar F, Arpinati M, Paulli M, Maeda T, Gibellini D, Pagano L, Pimpinelli N, Santucci M, Cerroni L, Croce CM, Facchetti F, Piccaluga PP, Pileri SA, AIRC 5xMille consortium ‘Genetics-driven targeted management of lymphoid malignancies and the Italian Registry on Blastic Plasmacytoid Dendritic Cell Neoplasm. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 2014 Aug:28(8):1606-16. doi: 10.1038/leu.2014.64. Epub 2014 Feb 7
[PubMed PMID: 24504027]
[12]
Rodrigues PF, Alberti-Servera L, Eremin A, Grajales-Reyes GE, Ivanek R, Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nature immunology. 2018 Jul:19(7):711-722. doi: 10.1038/s41590-018-0136-9. Epub 2018 Jun 20
[PubMed PMID: 29925996]
[13]
Sapienza MR, Pileri A, Derenzini E, Melle F, Motta G, Fiori S, Calleri A, Pimpinelli N, Tabanelli V, Pileri S. Blastic Plasmacytoid Dendritic Cell Neoplasm: State of the Art and Prospects. Cancers. 2019 Apr 28:11(5):. doi: 10.3390/cancers11050595. Epub 2019 Apr 28
[PubMed PMID: 31035408]
[14]
Lee J, Zhou YJ, Ma W, Zhang W, Aljoufi A, Luh T, Lucero K, Liang D, Thomsen M, Bhagat G, Shen Y, Liu K. Lineage specification of human dendritic cells is marked by IRF8 expression in hematopoietic stem cells and multipotent progenitors. Nature immunology. 2017 Aug:18(8):877-888. doi: 10.1038/ni.3789. Epub 2017 Jun 26
[PubMed PMID: 28650480]
[15]
Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010 Dec 14:33(6):905-16. doi: 10.1016/j.immuni.2010.11.023. Epub 2010 Dec 9
[PubMed PMID: 21145760]
[16]
Ippolito GC, Dekker JD, Wang YH, Lee BK, Shaffer AL 3rd, Lin J, Wall JK, Lee BS, Staudt LM, Liu YJ, Iyer VR, Tucker HO. Dendritic cell fate is determined by BCL11A. Proceedings of the National Academy of Sciences of the United States of America. 2014 Mar 18:111(11):E998-1006. doi: 10.1073/pnas.1319228111. Epub 2014 Mar 3
[PubMed PMID: 24591644]
[17]
Kurotaki D, Kawase W, Sasaki H, Nakabayashi J, Nishiyama A, Morse HC 3rd, Ozato K, Suzuki Y, Tamura T. Epigenetic control of early dendritic cell lineage specification by the transcription factor IRF8 in mice. Blood. 2019 Apr 25:133(17):1803-1813. doi: 10.1182/blood-2018-06-857789. Epub 2019 Feb 22
[PubMed PMID: 30796024]
[18]
Bigley V, Maisuria S, Cytlak U, Jardine L, Care MA, Green K, Gunawan M, Milne P, Dickinson R, Wiscombe S, Parry D, Doffinger R, Laurence A, Fonseca C, Stoevesandt O, Gennery A, Cant A, Tooze R, Simpson AJ, Hambleton S, Savic S, Doody G, Collin M. Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. The Journal of allergy and clinical immunology. 2018 Jun:141(6):2234-2248. doi: 10.1016/j.jaci.2017.08.044. Epub 2017 Nov 8
[PubMed PMID: 29128673]
[19]
Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Advances in anatomic pathology. 2009 Nov:16(6):392-404. doi: 10.1097/PAP.0b013e3181bb6bc2. Epub
[PubMed PMID: 19851130]
Level 3 (low-level) evidence
[20]
Assaf C, Gellrich S, Whittaker S, Robson A, Cerroni L, Massone C, Kerl H, Rose C, Chott A, Chimenti S, Hallermann C, Petrella T, Wechsler J, Bagot M, Hummel M, Bullani-Kerl K, Bekkenk MW, Kempf W, Meijer CJ, Willemze R, Sterry W. CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. Journal of clinical pathology. 2007 Sep:60(9):981-9
[PubMed PMID: 17018683]
[21]
Jain A, Sweet K. Blastic Plasmacytoid Dendritic Cell Neoplasm. Journal of the National Comprehensive Cancer Network : JNCCN. 2023 May:21(5):515-521. doi: 10.6004/jnccn.2023.7026. Epub
[PubMed PMID: 37156483]
[22]
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul:36(7):1703-1719. doi: 10.1038/s41375-022-01613-1. Epub 2022 Jun 22
[PubMed PMID: 35732831]
[23]
Pileri A, Delfino C, Grandi V, Agostinelli C, Pileri SA, Pimpinelli N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): the cutaneous sanctuary. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2012 Dec:147(6):603-8
[PubMed PMID: 23149706]
[24]
Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, Dictor M, Hansmann ML, Pileri SA, Dyer MJ, Sozzani S, Dikic I, Shaw AS, Petrella T, Stein H, Isaacson PG, Facchetti F, Mason DY. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008 Apr 1:111(7):3778-92. doi: 10.1182/blood-2007-10-117531. Epub 2008 Jan 24
[PubMed PMID: 18218851]
[25]
Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood. 2003 Jun 15:101(12):5007-9
[PubMed PMID: 12576313]
[26]
Montes-Moreno S, Ramos-Medina R, Martínez-López A, Barrionuevo Cornejo C, Parra Cubillos A, Quintana-Truyenque S, Rodriguez Pinilla SM, Pajares R, Sanchez-Verde L, Martinez-Torrecuadrada J, Roncador G, Piris MA. SPIB, a novel immunohistochemical marker for human blastic plasmacytoid dendritic cell neoplasms: characterization of its expression in major hematolymphoid neoplasms. Blood. 2013 Jan 24:121(4):643-7. doi: 10.1182/blood-2012-08-447599. Epub 2012 Nov 19
[PubMed PMID: 23165482]
[27]
Petrella T, Meijer CJ, Dalac S, Willemze R, Maynadié M, Machet L, Casasnovas O, Vergier B, Teitell MA. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. American journal of clinical pathology. 2004 Aug:122(2):307-13
[PubMed PMID: 15323148]
[28]
Yun S, Chan O, Kerr D, Vincelette ND, Idrees A, Mo Q, Sweet K, Lancet JE, Kharfan-Dabaja MA, Zhang L, Sokol L. Survival outcomes in blastic plasmacytoid dendritic cell neoplasm by first-line treatment and stem cell transplant. Blood advances. 2020 Jul 28:4(14):3435-3442. doi: 10.1182/bloodadvances.2020001875. Epub
[PubMed PMID: 32722779]
Level 3 (low-level) evidence
[29]
Pemmaraju N, Konopleva M. Approval of tagraxofusp-erzs for blastic plasmacytoid dendritic cell neoplasm. Blood advances. 2020 Aug 25:4(16):4020-4027. doi: 10.1182/bloodadvances.2019000173. Epub
[PubMed PMID: 32841341]
Level 3 (low-level) evidence
[30]
Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomarker research. 2014 Feb 10:2(1):4. doi: 10.1186/2050-7771-2-4. Epub 2014 Feb 10
[PubMed PMID: 24513123]
[31]
Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, Rizzieri DA, Wang ES, Duvic M, Sloan JM, Spence S, Shemesh S, Brooks CL, Balser J, Bergstein I, Lancet JE, Kantarjian HM, Konopleva M. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. The New England journal of medicine. 2019 Apr 25:380(17):1628-1637. doi: 10.1056/NEJMoa1815105. Epub
[PubMed PMID: 31018069]
[32]
Hammond D, Pemmaraju N. Tagraxofusp for Blastic Plasmacytoid Dendritic Cell Neoplasm. Hematology/oncology clinics of North America. 2020 Jun:34(3):565-574. doi: 10.1016/j.hoc.2020.01.005. Epub 2020 Mar 18
[PubMed PMID: 32336420]
[33]
Taylor J, Haddadin M, Upadhyay VA, Grussie E, Mehta-Shah N, Brunner AM, Louissaint A Jr, Lovitch SB, Dogan A, Fathi AT, Stone RM, Tallman MS, Rampal RK, Neuberg DS, Stevenson KE, Horwitz SM, Lane AA. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood. 2019 Aug 22:134(8):678-687. doi: 10.1182/blood.2019001144. Epub 2019 Jun 26
[PubMed PMID: 31243042]
[34]
Kharfan-Dabaja MA, Reljic T, Murthy HS, Ayala E, Kumar A. Allogeneic Hematopoietic Cell Transplantation Is an Effective Treatment for Blastic Plasmacytoid Dendritic Cell Neoplasm in First Complete Remission: Systematic Review and Meta-analysis. Clinical lymphoma, myeloma & leukemia. 2018 Nov:18(11):703-709.e1. doi: 10.1016/j.clml.2018.07.295. Epub 2018 Aug 2
[PubMed PMID: 30145196]
Level 1 (high-level) evidence
[35]
Alfayez M, Konopleva M, Pemmaraju N. Role of tagraxofusp in treating blastic plasmacytoid dendritic cell neoplasm (BPDCN). Expert opinion on biological therapy. 2020 Feb:20(2):115-123. doi: 10.1080/14712598.2020.1701651. Epub 2019 Dec 17
[PubMed PMID: 31801379]
Level 3 (low-level) evidence
[36]
Abla D, Abboud MR, Noun D, Tarek N, Pemmaraju N. Hyper-CVAD combined with Venetoclax for relapsed pediatric blastic plasmacytoid dendritic cell neoplasm (BPDCN): A case report and literature review. Leukemia research reports. 2022:17():100313. doi: 10.1016/j.lrr.2022.100313. Epub 2022 Apr 12
[PubMed PMID: 35462725]
Level 3 (low-level) evidence
[38]
Ono K, Ise M, Ikebe D, Sato A, Wang X, Sugawara T, Tsujimura H, Itami M, Kumagai K. [Successful treatment with biweekly CHOP for bone marrow relapse of blastic plasmacytoid dendritic cell neoplasm]. [Rinsho ketsueki] The Japanese journal of clinical hematology. 2017:58(2):150-154. doi: 10.11406/rinketsu.58.150. Epub
[PubMed PMID: 28321093]
[39]
Montero J, Stephansky J, Cai T, Griffin GK, Cabal-Hierro L, Togami K, Hogdal LJ, Galinsky I, Morgan EA, Aster JC, Davids MS, LeBoeuf NR, Stone RM, Konopleva M, Pemmaraju N, Letai A, Lane AA. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer discovery. 2017 Feb:7(2):156-164. doi: 10.1158/2159-8290.CD-16-0999. Epub 2016 Dec 16
[PubMed PMID: 27986708]
[40]
Bétrian S, Guenounou S, Luquet I, Demur C, Huynh A, Ysebaert L, Recher C, Huguet F. Bendamustine for relapsed blastic plasmacytoid dendritic cell leukaemia. Hematological oncology. 2017 Jun:35(2):252-255. doi: 10.1002/hon.2252. Epub 2015 Oct 8
[PubMed PMID: 28620927]
[41]
Zalmaï L, Viailly PJ, Biichle S, Cheok M, Soret L, Angelot-Delettre F, Petrella T, Collonge-Rame MA, Seilles E, Geffroy S, Deconinck E, Daguindau E, Bouyer S, Dindinaud E, Baunin V, Le Garff-Tavernier M, Roos-Weil D, Wagner-Ballon O, Salaun V, Feuillard J, Brun S, Drenou B, Mayeur-Rousse C, Okamba P, Dorvaux V, Tichionni M, Rose J, Rubio MT, Jacob MC, Raggueneau V, Preudhomme C, Saas P, Ferrand C, Adotevi O, Roumier C, Jardin F, Garnache-Ottou F, Renosi F. Plasmacytoid dendritic cells proliferation associated with acute myeloid leukemia: phenotype profile and mutation landscape. Haematologica. 2021 Dec 1:106(12):3056-3066. doi: 10.3324/haematol.2020.253740. Epub 2021 Dec 1
[PubMed PMID: 33054115]
[42]
Fei F, Liedtke M, Silva O. Case Report: Mature Plasmacytoid Dendritic Cell Proliferation Associated With a Lymphoid Neoplasm. Frontiers in oncology. 2022:12():903113. doi: 10.3389/fonc.2022.903113. Epub 2022 Jul 6
[PubMed PMID: 35875095]
Level 3 (low-level) evidence
[43]
Sukswai N, Aung PP, Yin CC, Li S, Wang W, Wang SA, Ortega V, Lyapichev K, Nagarajan P, Alfattal R, Angelova E, Tang Z, Loghavi S, Kanagal-Shamanna R, Miranda RN, Pemmaraju N, Bhalla K, Konopleva M, Medeiros LJ, Khoury JD. Dual Expression of TCF4 and CD123 Is Highly Sensitive and Specific For Blastic Plasmacytoid Dendritic Cell Neoplasm. The American journal of surgical pathology. 2019 Oct:43(10):1429-1437. doi: 10.1097/PAS.0000000000001316. Epub
[PubMed PMID: 31261288]
[44]
Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, Pappa V, Marinakis T, Anagnostopoulos NI, Vadikolia C, Anagnostopoulos A, Angelopoulou MK, Terpos E, Poziopoulos C, Anargyrou K, Rontogianni D, Papadaki T, Psarra A, Kontopidou FN, Skoumi D, Papadhimitriou SI, Paterakis G, Hellenic Dendritic Cell Leukemia Study Group. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leukemia research. 2010 Apr:34(4):438-46. doi: 10.1016/j.leukres.2009.09.006. Epub 2009 Sep 29
[PubMed PMID: 19793612]
[45]
Hashikawa K, Niino D, Yasumoto S, Nakama T, Kiyasu J, Sato K, Kimura Y, Takeuchi M, Sugita Y, Hashimoto T, Ohshima K. Clinicopathological features and prognostic significance of CXCL12 in blastic plasmacytoid dendritic cell neoplasm. Journal of the American Academy of Dermatology. 2012 Feb:66(2):278-91. doi: 10.1016/j.jaad.2010.12.043. Epub 2011 Aug 11
[PubMed PMID: 21835496]
[46]
Jegalian AG, Buxbaum NP, Facchetti F, Raffeld M, Pittaluga S, Wayne AS, Jaffe ES. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica. 2010 Nov:95(11):1873-9. doi: 10.3324/haematol.2010.026179. Epub 2010 Jul 27
[PubMed PMID: 20663945]
[47]
Jaye DL, Geigerman CM, Herling M, Eastburn K, Waller EK, Jones D. Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Dec:19(12):1555-62
[PubMed PMID: 16998465]