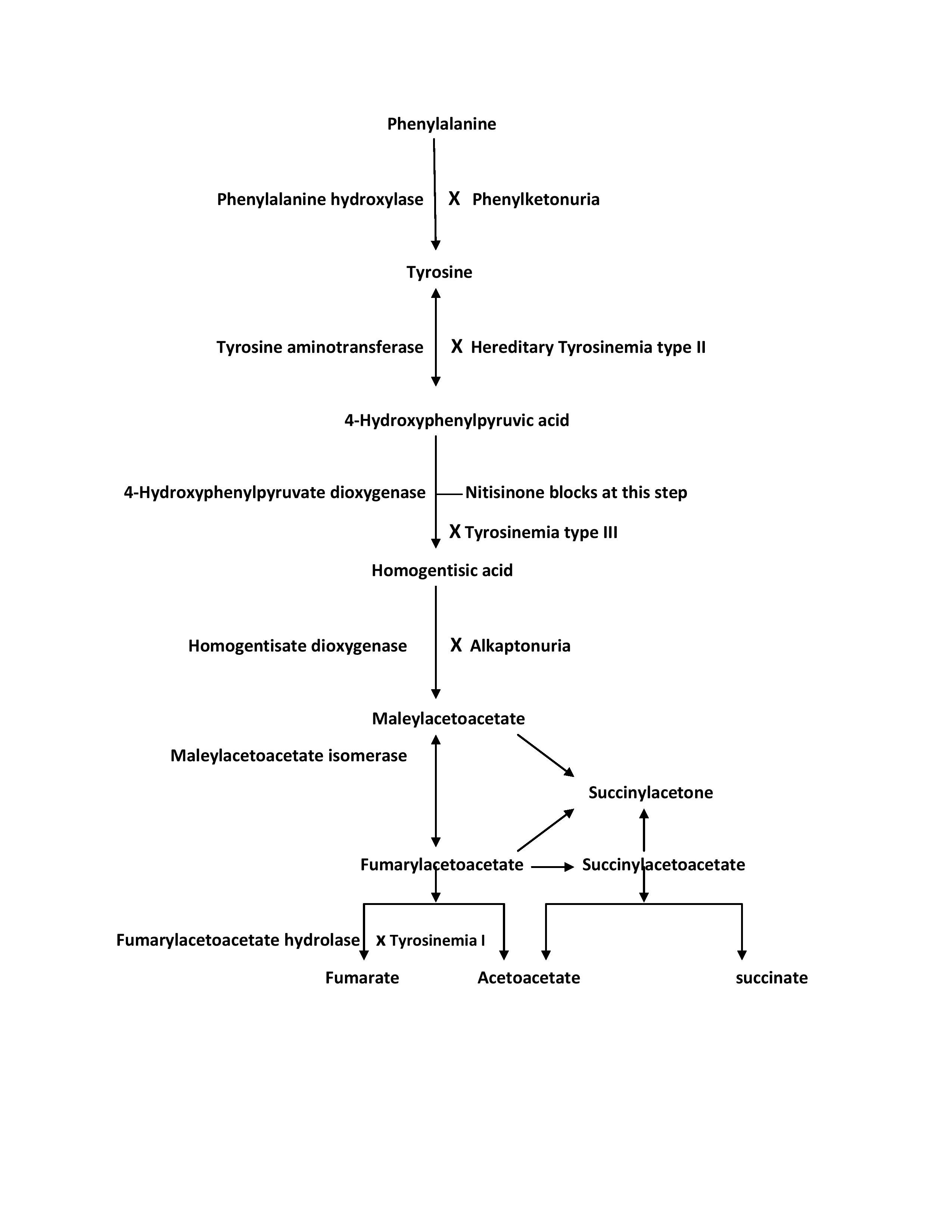
[1]
Cannon Homaei S,Barone H,Kleppe R,Betari N,Reif A,Haavik J, ADHD symptoms in neurometabolic diseases: Underlying mechanisms and clinical implications. Neuroscience and biobehavioral reviews. 2021 Nov 11
[PubMed PMID: 34774900]
[2]
Mitchell G,Larochelle J,Lambert M,Michaud J,Grenier A,Ogier H,Gauthier M,Lacroix J,Vanasse M,Larbrisseau A, Neurologic crises in hereditary tyrosinemia. The New England journal of medicine. 1990 Feb 15;
[PubMed PMID: 2153931]
[3]
Chinsky JM,Singh R,Ficicioglu C,van Karnebeek CDM,Grompe M,Mitchell G,Waisbren SE,Gucsavas-Calikoglu M,Wasserstein MP,Coakley K,Scott CR, Diagnosis and treatment of tyrosinemia type I: a US and Canadian consensus group review and recommendations. Genetics in medicine : official journal of the American College of Medical Genetics. 2017 Dec;
[PubMed PMID: 28771246]
Level 3 (low-level) evidence
[4]
Menon J,Shanmugam N,Valamparampil JJ,Hakeem A,Vij M,Jalan A,Reddy MS,Rela M, Liver Transplantation: A Safe and Definitive Alternative to Lifelong Nitisinone for Tyrosinemia Type 1. Indian journal of pediatrics. 2021 Aug 16;
[PubMed PMID: 34398413]
[5]
Phaneuf D,Labelle Y,Bérubé D,Arden K,Cavenee W,Gagné R,Tanguay RM, Cloning and expression of the cDNA encoding human fumarylacetoacetate hydrolase, the enzyme deficient in hereditary tyrosinemia: assignment of the gene to chromosome 15. American journal of human genetics. 1991 Mar;
[PubMed PMID: 1998338]
[6]
Manning K,Al-Dhalimy M,Finegold M,Grompe M, In vivo suppressor mutations correct a murine model of hereditary tyrosinemia type I. Proceedings of the National Academy of Sciences of the United States of America. 1999 Oct 12;
[PubMed PMID: 10518553]
[7]
Stinton C,Geppert J,Freeman K,Clarke A,Johnson S,Fraser H,Sutcliffe P,Taylor-Phillips S, Newborn screening for Tyrosinemia type 1 using succinylacetone - a systematic review of test accuracy. Orphanet journal of rare diseases. 2017 Mar 9;
[PubMed PMID: 28274233]
Level 1 (high-level) evidence
[8]
Locatelli F,Puzenat E,Arnoux JB,Blanc D,Aubin F, Richner-Hanhart syndrome (tyrosinemia type II). Cutis. 2017 Dec;
[PubMed PMID: 29360903]
[9]
Natt E,Kida K,Odievre M,Di Rocco M,Scherer G, Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proceedings of the National Academy of Sciences of the United States of America. 1992 Oct 1;
[PubMed PMID: 1357662]
[10]
Hühn R,Stoermer H,Klingele B,Bausch E,Fois A,Farnetani M,Di Rocco M,Boué J,Kirk JM,Coleman R,Scherer G, Novel and recurrent tyrosine aminotransferase gene mutations in tyrosinemia type II. Human genetics. 1998 Mar;
[PubMed PMID: 9544843]
[11]
Giardini O,Cantani A,Kennaway NG,D'Eufemia P, Chronic tyrosinemia associated with 4-hydroxyphenylpyruvate dioxygenase deficiency with acute intermittent ataxia and without visceral and bone involvement. Pediatric research. 1983 Jan;
[PubMed PMID: 6132360]
[12]
Rüetschi U,Cerone R,Pérez-Cerda C,Schiaffino MC,Standing S,Ugarte M,Holme E, Mutations in the 4-hydroxyphenylpyruvate dioxygenase gene (HPD) in patients with tyrosinemia type III. Human genetics. 2000 Jun;
[PubMed PMID: 10942115]
[13]
Levine SZ,Marples E,Gordon HH, A DEFECT IN THE METABOLISM OF AROMATIC AMINO ACIDS IN PREMATURE INFANTS: THE ROLE OF VITAMIN C. Science (New York, N.Y.). 1939 Dec 29;
[PubMed PMID: 17792637]
[14]
Kaye CI,Committee on Genetics.,Accurso F,La Franchi S,Lane PA,Hope N,Sonya P,G Bradley S,Michele A LP, Newborn screening fact sheets. Pediatrics. 2006 Sep;
[PubMed PMID: 16950973]
[15]
De Braekeleer M,Larochelle J, Genetic epidemiology of hereditary tyrosinemia in Quebec and in Saguenay-Lac-St-Jean. American journal of human genetics. 1990 Aug;
[PubMed PMID: 2378355]
[16]
Kawabata K,Kido J,Yoshida T,Matsumoto S,Nakamura K, A case report of two siblings with hypertyrosinemia type 1 presenting with hepatic disease with different onset time and severity. Molecular genetics and metabolism reports. 2022 Sep;
[PubMed PMID: 35800472]
Level 3 (low-level) evidence
[17]
Forget S,Patriquin HB,Dubois J,Lafortune M,Merouani A,Paradis K,Russo P, The kidney in children with tyrosinemia: sonographic, CT and biochemical findings. Pediatric radiology. 1999 Feb;
[PubMed PMID: 9933329]
[18]
al-Essa MA,Rashed MS,Ozand PT, Tyrosinaemia type II: an easily diagnosed metabolic disorder with a rewarding therapeutic response. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 1999 Nov;
[PubMed PMID: 11924112]
[19]
Heylen E,Scherer G,Vincent MF,Marie S,Fischer J,Nassogne MC, Tyrosinemia Type III detected via neonatal screening: management and outcome. Molecular genetics and metabolism. 2012 Nov;
[PubMed PMID: 23036342]
[20]
Najafi R,Mostofizadeh N,Hashemipour M, A Case of Tyrosinemia Type III with Status Epilepticus and Mental Retardation. Advanced biomedical research. 2018;
[PubMed PMID: 29456978]
Level 3 (low-level) evidence
[21]
Tang Y,Kong Y, Hereditary tyrosinemia type Ⅰ: newborn screening, diagnosis and treatment. Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences. 2021 Aug 25;
[PubMed PMID: 34704422]
[22]
Lindstedt S,Holme E,Lock EA,Hjalmarson O,Strandvik B, Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet (London, England). 1992 Oct 3;
[PubMed PMID: 1383656]
[23]
Masurel-Paulet A,Poggi-Bach J,Rolland MO,Bernard O,Guffon N,Dobbelaere D,Sarles J,de Baulny HO,Touati G, NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. Journal of inherited metabolic disease. 2008 Feb;
[PubMed PMID: 18214711]
[24]
Larochelle J,Alvarez F,Bussières JF,Chevalier I,Dallaire L,Dubois J,Faucher F,Fenyves D,Goodyer P,Grenier A,Holme E,Laframboise R,Lambert M,Lindstedt S,Maranda B,Melançon S,Merouani A,Mitchell J,Parizeault G,Pelletier L,Phan V,Rinaldo P,Scott CR,Scriver C,Mitchell GA, Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Molecular genetics and metabolism. 2012 Sep;
[PubMed PMID: 22885033]
[25]
van Ginkel WG,van Reemst HE,Kienstra NS,Daly A,Rodenburg IL,MacDonald A,Burgerhof JGM,de Blaauw P,van de Krogt J,Santra S,Heiner-Fokkema MR,van Spronsen FJ, The Effect of Various Doses of Phenylalanine Supplementation on Blood Phenylalanine and Tyrosine Concentrations in Tyrosinemia Type 1 Patients. Nutrients. 2019 Nov 18;
[PubMed PMID: 31752110]
[26]
Spiekerkoetter U,Couce ML,Das AM,de Laet C,Dionisi-Vici C,Lund AM,Schiff M,Spada M,Sparve E,Szamosi J,Vara R,Rudebeck M, Long-term safety and outcomes in hereditary tyrosinaemia type 1 with nitisinone treatment: a 15-year non-interventional, multicentre study. The lancet. Diabetes
[PubMed PMID: 34023005]
[27]
Yilmaz O,Daly A,Pinto A,Ashmore C,Evans S,Gupte G,Santra S,Preece MA,Mckiernan P,Kitchen S,Ayhan NY,MacDonald A, Natural Protein Tolerance and Metabolic Control in Patients with Hereditary Tyrosinaemia Type 1. Nutrients. 2020 Apr 19;
[PubMed PMID: 32325917]
[28]
Saudubray JM,Garcia-Cazorla À, Inborn Errors of Metabolism Overview: Pathophysiology, Manifestations, Evaluation, and Management. Pediatric clinics of North America. 2018 Apr;
[PubMed PMID: 29502909]
Level 3 (low-level) evidence
[29]
Bökenkamp A,Ludwig M, The oculocerebrorenal syndrome of Lowe: an update. Pediatric nephrology (Berlin, Germany). 2016 Dec;
[PubMed PMID: 27011217]
[30]
Kim MS,Moon JS,Kim MJ,Seong MW,Park SS,Ko JS, Hereditary Fructose Intolerance Diagnosed in Adulthood. Gut and liver. 2021 Jan 15;
[PubMed PMID: 33028743]