Introduction
An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. This quantity can be expressed as osmolality (in mmol/kg) or osmolarity (in mmol/L). Clinical laboratories typically measure osmolality, which is considered more precise as weight is temperature-independent.[1] In laboratory analysis, a dissolved substance is referred to as a solute, and the substance in which the solute is dissolved is referred to as a solvent. A solute dissolved into a solvent creates a solution. The unit for osmolar concentration or osmolality is milliosmole (abbreviated mOsm or mOsmol). For nonelectrolytes, 1 mmol equals 1 mOsm, whereas for electrolytes, the number of particles in a solution depends on the electrolyte's dissociation.[2][3]
When a solute is dissolved into a solvent, 4 colligative properties of the solvent change. These properties include:
- Osmotic pressure
- Vapor pressure
- Boiling point
- Freezing point
These properties are tied to osmolality and depend on the solution's number of solute particles. Dissolving a solute into a solvent generally increases the osmotic pressure and boiling point and decreases a solution's vapor pressure and freezing point. Although any of the 4 colligative properties could be used to determine the osmolality of a solution, technical limitations restrict the commercial measurement of osmolality to freezing point, vapor pressure, and membrane osmometers. The freezing point depression method is commonly employed in clinical laboratories due to its high accuracy and ease of execution.[4]
The general principle of freezing point depression osmometers involves the relationship between the number of moles of dissolved solute in a solution and the change in freezing point. For example, 1 mole of a dissolved solute reduces the freezing point of water by approximately 1.86 °C (~35.35 °F).[5] Therefore, freezing and thawing a solution and comparing the relative change in freezing point to that of a pure solvent allows for determining the approximate number of moles of dissolved solute in a sample. In clinical laboratory analysis, most samples are in water-based aqueous solutions, and the reference for solutions is generally pure water.
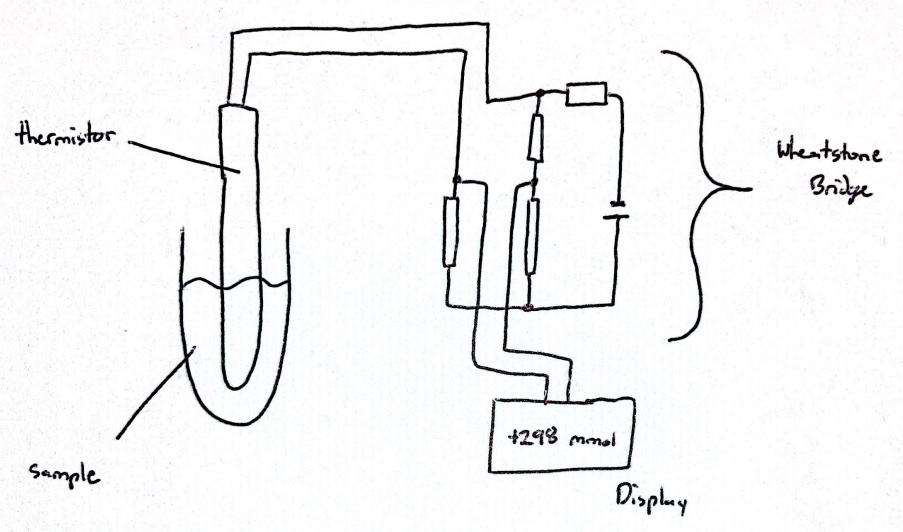
The setup of a freezing point depression osmometer includes a temperature-controlled bath to allow for sub-freezing temperatures, a thermistor probe connected to a Wheatstone bridge circuit to measure the temperature of a clinical sample, and a thermistor readout circuit, which represents a combination of a galvanometer and a potentiometer (see Image. Circuit Diagram of an Osmometer).[5][6] Although many osmometers are standalone tabletop devices, there are computerized, automatic, and handheld osmometers available (see Image. Computerized Osmometer).[7] Vapor pressure osmometers determine the osmolality of a solution by measuring the voltage difference between 2 thermistors. One thermistor is exposed to a sample solution, whereas the other is exposed to only the pure solvent corresponding to the sample solution. This method establishes a correlation between voltage and solute concentration, enabling the determination of the sample's osmolality.[8][9]
On the other hand, membrane osmometers measure the flow of solvent, often water, from a pure solvent container across a semipermeable membrane into a solution containing a solute and the same solvent. The flow across the semipermeable membrane can be measured as the osmotic pressure of a sample and is related to the concentration of solute in the sample solution.[1][10] The semipermeable membrane in this device only allows the flow of solvent and blocks the flow of dissolved particles. These osmometers are limited in their application by the range of osmolality they can measure and the membrane material with which the semipermeable membrane can be made.
Alternative methods to osmometers can be used to measure osmolality, including electrical conductivity and specific gravity.[11] Specific gravity was utilized before osmometers were practical to measure the osmolality of urine, utilizing various instruments such as refractometers, which measure the refraction of light through a fluid, or hydrometers. The applicability and accuracy of these methods besides osmometry for determining a sample's osmolality depend on the specific method and the sample.[8][9][10][11]