Continuing Education Activity
Renal infarction is the compromise of arterial blood supply to the kidney resulting in cell necrosis and loss of renal function. It has several causes, such as thrombo-emboli originating from the heart or in situ thrombosis. Patients typically present with flank pain; some may have microscopic or frank hematuria. Patients may have a history of atrial fibrillation, where cardiac thrombi can dislodge and eventually infarct the renal artery or one of its branches. Interventional procedures like angiography can result in embolic renal infarctions by causing an injury to the endothelium or dislodging an atherosclerotic plaque. Complications involve loss of renal function, which becomes evident in the form of declining GFR and increasing creatinine. This activity describes the investigation and management options of renal infarction by the interprofessional team.
Objectives:
- Identify the etiology of renal infarction.
- Review the appropriate evaluation of renal infarction.
- Describe the various management options in a case of renal infarction.
- Outline the follow-up care of patients with renal infarction.
Introduction
Renal infarction is a rare ischemic event or insult caused by the complete or partial occlusion of the main renal artery or its segmental branches, which may ultimately lead to the ischemic necrosis of renal tissue. It most commonly occurs due to an embolus originating from the heart or an in-situ thrombosis. Patients may have a history of atrial fibrillation, where cardiac thrombi can dislodge and eventually infarct the renal artery or one of its branches. Interventional procedures like angiography can result in embolic renal infarctions by causing an injury to the endothelium or dislodging an atherosclerotic plaque.[1]
Other etiological promotors of renal infarction are coagulation disorders, vasculitis, connective tissue diseases, valvular endocarditis (native or prosthetic valve), atherosclerosis of the aorta or renal artery, aortic aneurysms, smoking, and trauma.
Renal infarction patients typically present with abdominal or flank pain, nausea, vomiting, or fever. Gross or microscopic hematuria is often present. With the advent of contrast-enhanced computed tomography (CT) scans and other imaging modalities, more incidental cases of renal infarction are being detected in patients presenting with non-specific symptoms.
Often an underreported diagnosis, renal infarction patients can present late, or the diagnosis can be missed.[2] An overlooked diagnosis can result in irreversible loss of renal function, as renal reperfusion therapy is ineffective in a substantially delayed presentation.[3] In a study by Korzets et al., the time from admission to diagnosis ranged from 24 hours to 6 days.[4] Lessman et al. reported that only 4 out of 17 cases they studied were diagnosed correctly at initial presentation.[5]
The diagnosis of renal infarction should be considered in patients who develop sudden abdominal or flank pain with reduced renal function, hematuria, elevated LDH, or proteinuria and who do not have urolithiasis or any other diagnosable explanation for their symptoms. The chances increase if the patient has cardiac disease, especially atrial fibrillation, or is older. Since many of these patients will receive an initial non-contrast abdominal CT scan, progressing immediately to a contrast-enhanced study if the original CT scan is negative is quite reasonable and facilitates arriving at the correct diagnosis much more rapidly.[6][7] This is essential for initiating revascularization therapy promptly, which optimizes renal functional recovery.
Etiology
Bourgault et al. classified the etiology of renal infarction into four different groups in their retrospective study conducted between 1989 to 2011. In the study, the mean age of patients was highest in the cardiac group (65 ± 15 years) compared to other groups.[8] The etiology of renal infarction was cardioembolic in 28% and renal arterial abnormalities in 22%, as per a study by Mesiano et al.[9]
The causes of renal infarction were grouped into the following categories (i) cardioembolic origin, (ii) associated with renal artery injury, (iii) associated with hypercoagulability disorders, and (iv) idiopathic.
The most common cause of renal infarcts from a comprehensive metanalysis of the literature is cardioembolic disease which was responsible for 45.4% of the reported cases.[10] Within this group, 75.5% had atrial fibrillation.[10] Renal infarction may be the first sign of atrial fibrillation. Also, many patients with atrial fibrillation who develop renal infarction are found to be sub-therapeutic with anticoagulation.[11] Renal infarction due to embolic causes tends to occur in the older age group, often in the sixth to eighth decade of life.
Other cardiac causes include thrombo-emboli originating from the heart due to rheumatic mitral stenosis, myocardial infarction, prosthetic valve endocarditis, and infective endocarditis developing on valvular vegetations. Renal infarction can also occur from an embolus originating from an atrial myxoma or the apex of the left ventricle. Arterial causes of renal infarction include a thrombus originating from the aorta or renal artery in conditions such as atherosclerosis and an aortic dissection extending into the renal artery. Aneurysms of the thoracic or suprarenal abdominal aorta can increase the risk of renal infarctions by favoring the development of thrombi.[12]
Renal infarction can occur from a blunt abdominal injury leading to dissection or occlusion of the renal artery. Other causes of renal infarction include connective tissue disorders such as fibromuscular dysplasia, Marfan syndrome, Ehler-Danlos syndrome, and vasculitis disorders like polyarteritis nodosa.[8] Patients with COVID-19 are at higher risk of thromboembolic events, including renal infarctions.[13]
Fibromuscular dysplasia usually occurs in patients younger than age 50 without any known cardiovascular risk factors. Renal infarctions can happen due to thrombus formation in the post-stenotic, dilated portion of the artery. It usually manifests initially as hypertension.[14] Renal artery dissection can occur spontaneously in fibromuscular dysplasia and in Ehler-Danlos syndrome, which is associated with thrombotic aneurysms of the renal artery that may result in infarctions.[15][16]
Coagulation disorders like protein C and protein S deficiency, hyperhomocysteinemia, lupus anticoagulant, polycythemia vera, Factor V Leiden mutation, prothrombin II gene mutation, and antithrombin III deficiency can also lead to renal infarction. Idiopathic causes include all patients in whom a complete cardiac workup and coagulation panels have failed to reveal a cause of renal infarction and account for about 28.7% of cases (27 out of 94 patients), as described by Bourgault.[8] Patients in the idiopathic group were younger than those with cardiogenic renal infarction (median age 48 vs. 75, p < 0.003).[11][17]
Renal infarction may also be seen in women using oral contraceptives or associated with covid 19 infection, malignancy, sickle cell disease, anabolic steroid abuse, and cocaine use.[18] It can also be seen in renal diseases like glomerulonephritis, nephrotic syndrome, and deep vein thrombosis (DVT) with patent foramen ovale. Iatrogenic causes of renal infarction include endovascular or surgical procedures involving the aorta or its branches.
Coronary angiography is the most common procedure producing an embolic atherosclerotic plaque resulting in a renal infarction. It is responsible for about 80% of the iatrogenic cases from angiography.[19] Patients tend to be older, male, hypertensive, and diabetic with a smoking history. Symptoms are generally less severe. This is because atheroemboli have a solid but irregular shape and do not typically cause complete occlusion like thromboemboli.[20] This causes secondary ischemic atrophy, which over time, develops a foreign body reaction with giant cell formation and intimal proliferation, resulting in a narrowed arterial lumen and some degree of renal injury appearing over several weeks.[21]
A brachial angiographic approach is less risky than iliofemoral access as the aorta has the most atherosclerotic plaques susceptible to possible embolization.[22] Patients who undergo renal angiography often have diffuse atherosclerotic disease and are at a particularly high risk of renal atherosclerotic embolism, with an incidence of about 2%.[22]
Cholesterol crystal embolization of the renal artery is iatrogenic in 70% of cases, usually from the manipulation of larger arteries.[19][23]
Summary
- Cardiac problems, particularly atrial fibrillation, are the most common cause of renal infarcts, with a median age of 65.[10][24]
- Renal artery injury and a hypercoagulable state are the next most common causes of renal infarction, where the median age is 43 and 63 years, respectively.[24]
- Angiography, angioplasty, cardiovascular surgery, anticoagulation, and thrombolytic therapy are common causes of renal artery atheroembolism.[1]
- About 20% to 30% of cases remain idiopathic, with a median age of about 50.[8][10][24]
Epidemiology
The first case of renal embolic disease was reported in 1856.[5] The incidence of renal infarction in patients visiting the ED every year has been reported as 0.007% in a retrospective study conducted by Domanovits et al. during a 36-month observation period.[25]
In a study by Huang et al., the incidence of renal infarction was 0.004% (20 of 481,540), with the most common etiology being cardiac in origin (75%), i.e., 15 of 20 patients had atrial fibrillation, cardiac thrombus, or valvular heart disease.[26] In the same study, right-sided renal infarctions were more common than a left-sided and bilateral disease.[26]
In a study by Yang et al., unilateral renal involvement was seen in 80.9%, and bilateral involvement in 19.1% of patients.[27] It is generally believed that the reported incidence of renal infarction grossly underestimates its actual incidence as the presenting symptoms closely mimic those of acute renal colic and pyelonephritis, so it is often misdiagnosed. The detection rate of post-mortem renal infarctions is much higher at 1.4%.[28]
The mean age of patients who presented with renal infarction was 63.5 +/-15.42 years.[27] In a 10-year retrospective case series and review of literature of 165 patients presenting to the emergency department conducted by Antopolsky et al., there was no significant difference in sex distribution in renal infarction cases.[15]
The majority of patients who receive angiography have significant atherosclerotic disease. This puts them at higher risk for atherosclerotic plaque emboli resulting in renal artery occlusion and infarction at an overall rate of about 2%. This may be a relatively common cause of acute kidney injury, as renal atheroemboli have been reported in 7% of such patients.[29] This risk can be reduced using a brachial rather than an iliofemoral approach.[22]
Pathophysiology
Risk factors of renal infarction are atrial fibrillation, history of a previous embolism, mitral stenosis, hypertension, diabetes, and ischemic cardiac disease, as mentioned in a study by Domanovits.[25] These conditions lead to endothelial injury, which activates in-situ thrombus formation.
Another mechanism is a thrombus from the heart in atrial fibrillation, becoming dislodged and eventually occluding the renal artery. All renal segmental arteries are end-arteries, so a complete or partial reduction in vascular flow due to embolic or thrombotic occlusion leads to renal ischemia and infarction with focal tissue necrosis of the kidney. The consequent impairment in renal function can manifest as elevated creatinine levels and decreased glomerular filtration rates (GFR), leading to acute renal injury, chronic kidney disease (CKD), and sometimes end-stage renal failure.
Atheroemboli are more solid and irregular than thrombus-based emboli, but the crystals are smaller. They are, therefore, more likely to produce an incomplete renal arterial blockage than thromboembolic emboli and tend to affect smaller branches.[20] This eventually causes a foreign body reaction with giant cell formation, narrowing of the arterial lumen, and reduced renal blood flow resulting in renovascular hypertension with a reduction in renal function.[21]
History and Physical
Patients with acute renal infarctions typically present with the sudden onset of abdominal or flank pain along with associated symptoms like nausea, vomiting, hematuria, and occasionally fever.[8] On examination, abdominal or loin tenderness may be present.[3] In a retrospective study of 94 patients by Bourgault et al., abdominal pain was present in 96.8%, nausea in 27.6%, and vomiting and fever each in 20.2%. The study found hypertension in 48% of patients (46 out of 94 patients) in the study.[8]
Acute loss of renal arterial supply can lead to new-onset, acute renin-mediated hypertension noted at the time of initial presentation of patients, which usually improves with treatment.[2] Unfortunately, this is often attributed to a pain response and frequently goes unrecognized. Mean systolic and diastolic arterial pressure in renal infarction patients was 147.7 +/- 18.2 mmHg and 83.2 +/- 9.5 mmHg, respectively, according to Mesiano et al.[9]
Some patients may not experience any symptoms, further contributing to the underdiagnosis of renal infarction. Asymptomatic renal infarction can often be detected as an incidental finding in some patients undergoing imaging for unrelated reasons.[30]
Unfortunately, the clinical presentation is often either too non-specific or suggestive of alternative, more common disorders such as ureteral colic, a "passed" stone, pyelonephritis, or gastroenteritis. This confusion often leads to a delay, with less than 50% of patients receiving the correct diagnosis within two days of their initial presentation.
Atherosclerotic renal emboli are suggested by the triad of:
- Recent (within six months) precipitating event (angiography)
- Acute or subacute kidney injury (which may appear several weeks after the actual injurious event)
- Skin color changes such as "blue toe" syndrome or livedo reticularis will be present in about one-third of cases[31][32]
Cholesterol crystals may be found in the retina (Hollenhorst plaques), so a fundoscopic examination is suggested in suspected cases of atherosclerotic emboli.[33]
Since these cases do not typically present with acute symptoms, a high index of suspicion is necessary for higher-risk patients. The risk factors for general atherosclerotic disease include male gender, significant smoking history, hypertension, hypercholesterolemia, diabetes, and older age.[23] Essentially, any older male patient with a cardiac history and/or hypertension who presents as pyelonephritis or renal colic where there is no infection or evidence of stones or hydronephrosis should be suspected of a possible acute renal infarct. If their LDH is significantly elevated, then a renal infarction is likely.
Evaluation
Laboratory findings in renal infarction include leukocytosis, macroscopic or microscopic hematuria, elevated C reactive protein (CRP), and very high Lactate Dehydrogenase (LDH).[4] Bourgault et al. reported leukocytosis in 72.3% and elevated CRP levels in 77.6% of patients with renal infarction.[8]
In a study by Gasparini et al., out of 20 patients whose urinalysis results were available, hematuria was present in 65%, and proteinuria was present in 70% of patients with renal infarctions.[34] However, a larger study of 438 patients suggested that hematuria was likely found in just 32% and proteinuria in only 12%.[24]
LDH is a marker of cell necrosis and a sensitive indicator of renal infarction, which can rise to 4 or 5 times the normal value with no similar rise in serum aminotransferases.[2] In the study by Huang et al., 95% (19 of 20) of patients with renal infarction demonstrated elevated LDH levels with a mean and standard deviation of 812.1+/- 569.4 U/L.[26] Domanovits reported that LDH was elevated in 94% of patients presenting with acute renal infarction, and hematuria was present in 71%.[25]
A marked increase in LDH, especially with normal aminotransferase, is considered strongly suggestive of renal infarction as this is not associated with either urolithiasis, acute renal colic, or pyelonephritis.[17][35] This degree of LDH elevation (typically >500 IU/L) found in renal infarction is only otherwise seen in myocardial infarction, hemolysis, and renal transplant rejection, which are all easily clinically distinguishable from renal infarctions.[35] Serum LDH is highly sensitive but not specific, so other causes of LDH elevation must be excluded. LDH can remain elevated up to 15 days after the first clinical presentation.[8]
Other possible lab findings in renal infarctions are abnormally elevated creatinine and creatine kinase values and a decline in GFR. The rise in creatinine is more evident in patients with a particularly large renal infarct or bilateral involvement of both kidneys.[8] Proteinuria may also be present in some cases. A urinalysis and culture can be done to look for hematuria and proteinuria and to eliminate some of the differential diagnoses, but it is often not helpful since the findings overlap with other conditions.
Atherosclerotic emboli causing a renal infarction would be associated with eosinophilia, eosinophiluria, and low serum complement in the acute phase. However, these findings disappear after about one week from the time of the injury.[36][37][38] Focal neurological defects, mental confusion, localized cyanosis, and retinal Hollenhorst plaques suggest atherosclerotic emboli.[1]
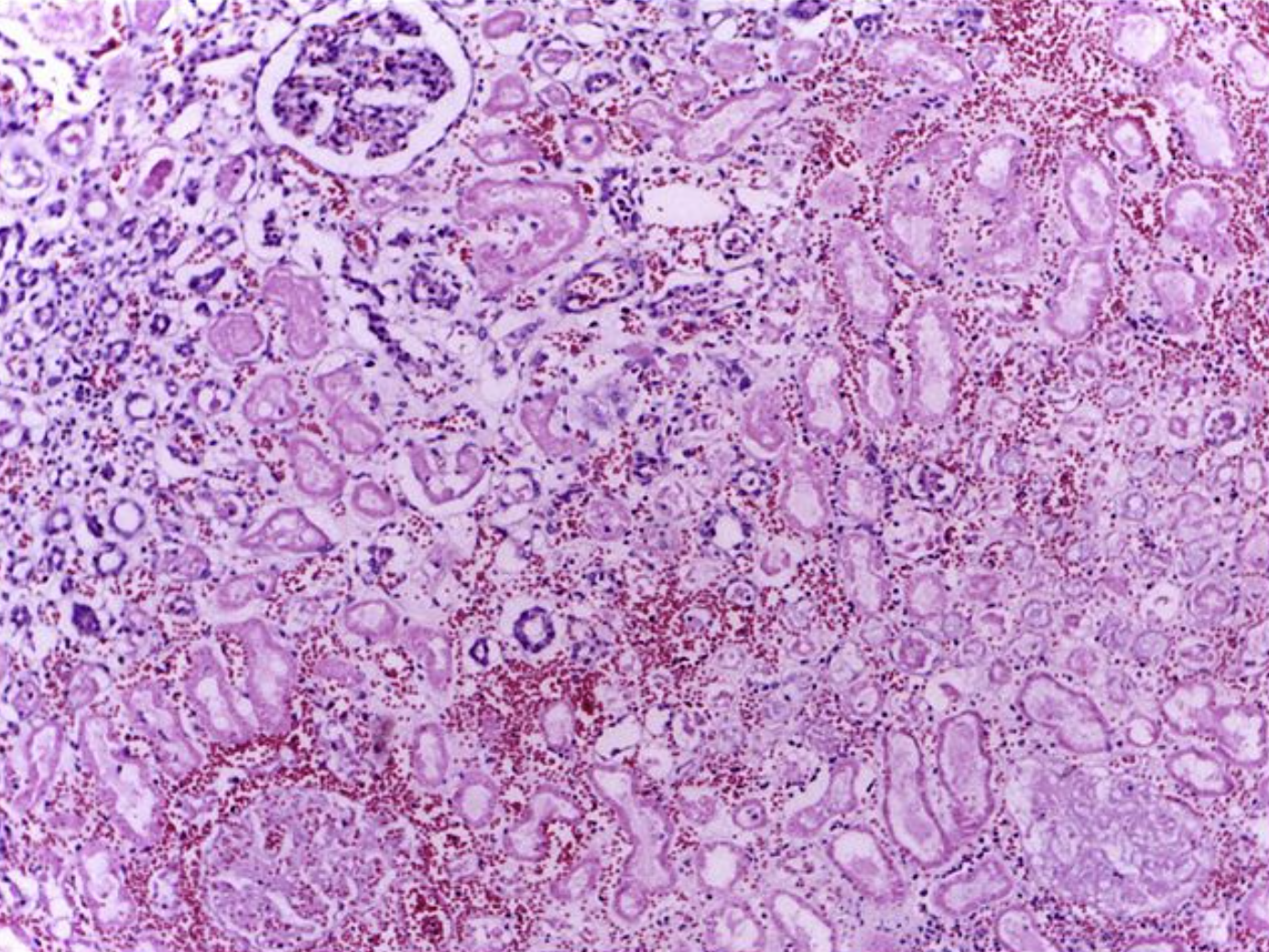
Atheroembolic renal emboli also tend to produce a slow and often subacute decrease in renal function, which typically plateaus at three to eight weeks.[21] A renal biopsy is often necessary for a definitive diagnosis of atheroembolic renal infarction. This will detect atheroemboli in over 75% of cases where intraluminal cholesterol crystals appear as bi-convex, needle-shaped defects, or "ghosts" as the cholesterol dissolved during the fixation process.[39] There is often significant vascular intimal inflammation and possibly eosinophilic infiltration.[37] Focal glomerulosclerosis may also be seen.[40] Only about 12% of cases are diagnosed correctly and in a timely fashion.[41]
Imaging
The initial investigation in patients presenting with acute flank pain, vomiting, and hematuria is usually a non-contrast CT scan of the abdomen and pelvis to rule out urolithiasis and pyelonephritis, which are the most common reasons for such a clinical presentation.[42] If a non-contrast CT of the abdomen and pelvis is non-contributory, a contrast-enhanced CT of the abdomen should be done to rule out renal infarction as a cause of flank pain.[15][16] Domanovits et al. suggested that a contrast-enhanced CT should be performed within 24 hours in all patients presenting with the triad of a high risk of thromboembolic events, persistent flank/abdominal/lower back pain, and high LDH levels and/or hematuria.[25]
The only findings of renal infarction in a non-contrast CT abdomen may be some perinephric stranding and mild renal swelling, but these can easily be misinterpreted as signs of a recently passed kidney stone. The standard test for a suspected renal infarction is a contrast-enhanced CT scan of the abdomen which should be done in cases of possible renal infarction with negative non-contrast studies, especially in patients with elevated LDH, atrial fibrillation, increased risk, or history of thrombosis, or elevated age (>70).
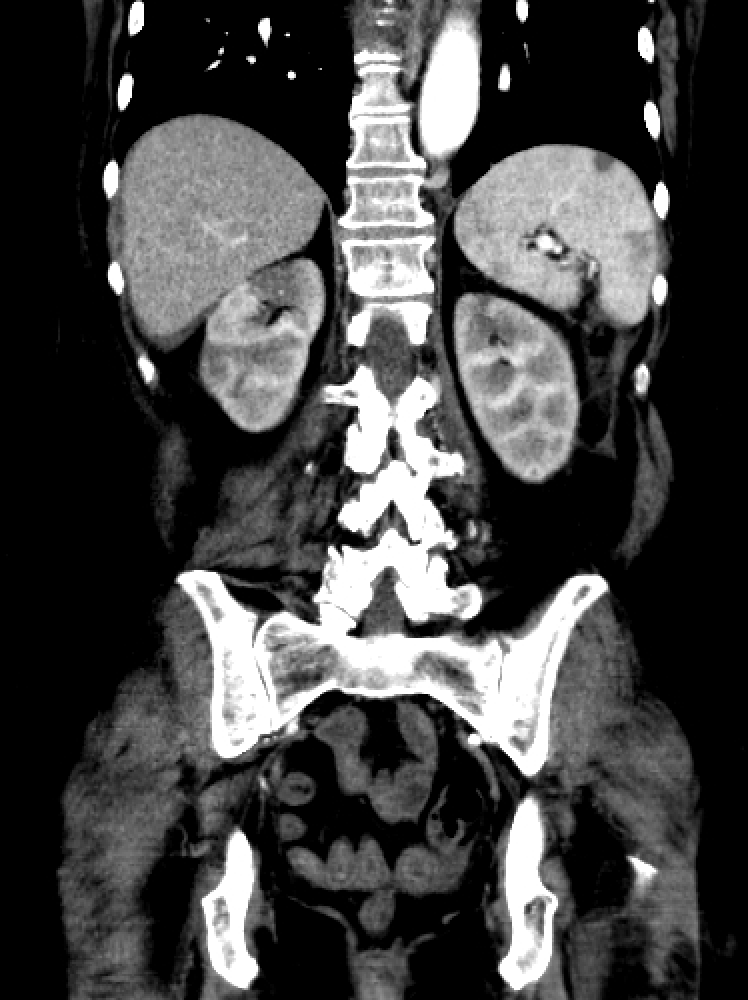
In an intravenous contrast-enhanced CT of the abdomen, a clear hypodensity or wedge-shaped perfusion defect can be appreciated, mostly in the arterial phase. In a segmental infarct, there may be a thin rim of the renal cortex paralleling the wedge-shaped area of the infarct, presenting with normal enhancement. This is called the cortical rim sign, which is seen in about half of the cases of renal infarction.[43][44] This sign may become evident several days after a renal infarction due to preserved perfusion of the capsule by perforating branches of the renal capsular artery receiving collateral circulation from branches of the suprarenal, ureteral and lumbar vessels.[45] If there is total occlusion of the main renal artery, there can be non-enhancement of the entire kidney. (In a traumatic transection of the renal artery, CT of the abdomen may reveal a hematoma starting at the hilum of the renal artery.)
Renal CT arteriography is the gold standard investigation for renal infarction.[15] It not only makes the definitive diagnosis but also allows for proper decision-making with regard to treatment.[15] Renal MRI arteriography is equally helpful, but the examination takes much longer to perform, so CT imaging is usually preferred. Other investigations used to confirm the diagnosis of renal infarction are magnetic resonance imaging (MRI) with gadolinium contrast excretion and DMSA radioisotope scans.
A color doppler scan may also be beneficial in demonstrating decreased or absent blood flow to the infarcted area. Contrast agents like gadolinium in MRI must be used after considering the patient's renal function as it poses a risk for developing nephrogenic systemic fibrosis. An intravenous pyelogram (IVP) in a renal infarction demonstrates non-visualization of the infarcted renal parenchyma. A DMSA radioisotope scan will demonstrate decreased or no tracer uptake in the affected area.[2]
No further radiological investigation may be necessary if the initial non-contrast CT depicts an atrophic and scarred kidney as would present in an old chronic renal infarction because interventions are unlikely to salvage the kidney at that point.
An EKG should be done to look for atrial fibrillation in all patients diagnosed with renal infarction. A transthoracic or transesophageal echocardiogram can evaluate the possibility of cardiac thromboembolic disease and thrombi. A careful assessment of coagulation disorders should be performed if cardiac and renal arterial pathology have been eliminated as causative factors of renal infarction or if surgical revascularization is being considered.[45]
There are clinical factors that might help distinguish renal infarction from renal colic.[46] They include:
- Age >70 years with no previous history of nephrolithiasis
- Atrial fibrillation
- C-reactive protein >0.23 mg/dL
- Serum LDH >500 IU/L (especially with normal serum aminotransferases)
- More likely to be diabetic and hypertensive
- Negative urinalysis for RBCs
- Negative non-contrast CT scan
Treatment / Management
CT renal angiography is usually performed to better evaluate the vasculature, identify the specific vessels involved, and determine the degree of occlusion. This is generally not necessary if the earlier diagnostic contrast-enhanced abdominal CT demonstrates a shrunken, atrophic kidney or a clearly demarcated, dense wedge-shaped scar suggesting an old infarction where the involved renal tissue would be unlikely to benefit from revascularization.
Treating renal infarction could be broadly categorized into catheter-directed thrombolysis, systemic thrombolysis, anticoagulation, and surgery. All patients are assessed for the benefit of revascularization procedures by considering the time since onset of ischemia, current kidney function, size and extent of the renal infarct, the presence of arterial dissection, the function of the contralateral kidney, the precise vessels involved, and whether the occlusion of the renal artery is partial or complete.
Revascularization is more likely to be beneficial if the patient has the following:
- Complete arterial occlusion of main or major segmental renal artery of <6 hours duration, solitary kidney, or significant reduction in renal function from baseline (usually <50 mL/min GFR).[47]
- Partial arterial occlusion of main or major segmental renal artery of <24 hours duration. If >24 hours duration, consider revascularization if the patient has renal failure, continuing symptoms (flank pain), or hypertension that is new or worsening.[48]
- A renal infarction associated with arterial dissection.[49]
An infarction of the main renal artery jeopardizes the function of the entire organ. Segmental arterial occlusion can lead to reduced function of a large portion of the kidney, which will be significant in patients with pre-existing renal failure or solitary kidneys. Blockage of a subsegmental artery is what leads to the typical wedge-shaped diffusion defect of the parenchyma seen on contrast-enhanced CT scans of the abdomen.
Determining the timing of the onset of the infarction may not always be simple. Acute symptoms, such as flank pain, nausea, vomiting, and hypertension, suggest a more recent onset of one week or less.
Patients with normal kidney function may not demonstrate any decrease in overall kidney function even with complete, acute unilateral renal arterial occlusion. However, a significant reduction in renal function would be expected in patients with pre-existing kidney disease, those who develop acute kidney injury, and those with bilateral complete renal infarctions.
The degree of renal arterial blockage can be determined by CT renal angiography. Partial occlusion with some degree of visualized parenchymal perfusion suggests potential recovery. Typically, even complete occlusions of 6 hours or less are considered potentially reversible.
Catheter-directed thrombolysis (also known as percutaneous endovascular therapy) is mostly attempted for revascularization in proximal or bilateral renal artery occlusions and to restore perfusion in a solitary kidney. It may also be considered for selected patients with significant segmental artery occlusions. Patients with underlying fibromuscular dysplasia should be referred for percutaneous transluminal angioplasty or vascular reconstructive surgery.
Catheter-directed thrombolysis in the form of local intraarterial thrombolysis, thrombectomy, angioplasty, and/or stenting is beneficial only in patients diagnosed early within the ischemic tolerance of normal kidneys.[50][51][52]
One published protocol attempts thrombectomy first; if unsuccessful, a bolus injection of 250,000 IU of urokinase is given directly into the affected artery. If results are not deemed adequate, the catheter is left in place, and a continuous infusion of urokinase at a rate of 50,000 IU per hour is utilized for up to 72 hours. Daily renal angiograms are done while the urokinase infusion is administered, and the patients are carefully monitored for possible complications. If the infarction remains unchanged after 72 hours, the treatment is deemed ineffective and discontinued.[53]
According to Blum et al., recovery of renal function is expected if revascularization is attempted within 90 to 120 minutes of symptom onset.[54]
While some studies have reported renal function loss within two hours of ischemia, other studies have shown successful revascularization for up to 96 hours after presentation.[47] In catheter-directed thrombolysis, selective cannulation of the renal artery is done, and thrombolytic agents like urokinase, streptokinase, or tissue plasminogen activator are instilled. Using streptokinase for renal artery embolism was demonstrated first in humans by Fischer et al.[55]
All patients are given continuous intraarterial heparin during the procedure. Follow-up angiograms are taken to look for any residual thrombi. Patients who undergo angioplasty and/or stenting typically receive three to six months of aspirin and clopidogrel.
In a comparative study by Silverberg et al., 13 out of 42 patients (31%) were treated with catheter-directed thrombolysis. A complete resolution of the thrombus was seen in 10 out of 13 patients (76%), and a partial solution in the rest.[47]
Two patients in this study required permanent hemodialysis despite catheter-directed thrombolysis, but they were recipients of kidney transplants. Others have reported similar results, with the vast majority of patients showing complete or at least partial resolution if diagnosed and treated promptly.[49][52][56] The complications of catheter-directed thrombolysis include bleeding, pseudoaneurysm formation, and acute mesenteric embolism with ischemia.[57]
The maximal duration of complete blockage from a renal infarct that would still benefit from catheter-directed thrombolysis is unknown but is most likely limited to several days at most, but anecdotal reports of longer durations have been reported.[58][59] Partial blockages are more likely to respond to delayed therapy.[48]
Ultrasound-enhanced catheter-directed thrombolysis may be an innovative treatment option in renal artery thrombosis, wherein the ultrasound waves allow better penetration of thrombolytic agents by acoustic streaming.[60] Following endovascular therapy, patients are maintained on aspirin and clopidogrel; the clopidogrel can be discontinued after three months.[60]
Systemic thrombolysis can be attempted if catheter-directed thrombolysis is not available.[61][62] However, the risk of systemic bleeding is higher in systemic fibrinolytic therapy than in catheter-directed thrombolysis.[2] Compared to systemic fibrinolysis, catheter-directed thrombolysis has the advantage of a lower dosage of thrombolytic agents and better deliverability to the target blood vessels.[60] Reperfusion injury is a potential complication of thrombolysis except in an already atrophic kidney.
Anticoagulation is considered the treatment of choice for patients who present several days or longer after their renal infarction, as revascularization therapy is not likely to be beneficial. Long-term anticoagulation is also initiated for those with underlying hypercoagulation disorders or atrial fibrillation. The duration of anticoagulation is typically at least six months if the patient does not have risk factors for repeat embolization like atrial fibrillation or other hypercoagulable disorders. Intravenous heparin, followed by warfarin, is the standard anticoagulation regimen, with a target INR of 2 to 3. (This increases in patients already on warfarin before their renal infarct, in which an INR of 2.5 to 3.5 is suggested.)
Apixaban (a Factor Xa inhibitor and a direct oral anticoagulant) may also be used. It has the benefit of lower renal clearance and does not require the routine monitoring associated with long-term warfarin therapy. If anticoagulants are stopped after six months, life-long aspirin has been recommended for renal infarction patients even if no specific hypercoagulable state has been identified. Aspirin may also benefit those with asymptomatic remote renal infarctions who present with an atrophic kidney.
Open surgery is mostly attempted in the event of trauma-related renal artery injury and aortic dissection extending into the renal artery. In 1987, Ouriel et al. commented that operative intervention is likely to be unfavorable in cases of embolic renal infarctions.[48] Various surgical options include arteriotomy with embolectomy, aorto-renal, ileo-renal, or splenorenal bypass procedures.[34]
For renovascular hypertension, which patients may develop in the first week after a renal infarction, an angiotensin-converting enzyme (ACE) inhibitor or blocker is suggested. This is due to the increased renin levels that are not uncommon after a renal infarction. The associated hypertension frequently subsides in the following weeks unless the patients have underlying essential hypertension.[30][53] Long-term management of high blood pressure should be the same as for any hypertensive patient.[53]
Long-term follow-up for patients with thromboembolic renal infarctions has not been standardized. It is reasonable to monitor for complications related to ongoing therapy, such as bleeding episodes, and to check renal function regularly. Permanent life-long aspirin therapy is typically recommended as well.[24][63]
Follow-up imaging is recommended, and some experts have suggested serial abdominal imaging (preferably with magnetic resonance angiography) at 6 and 12 months. If no lesions or problems are found, further surveillance imaging may not be necessary. Patients with underlying renal or cardiac issues will require closer and longer-term monitoring.
Treatment of atherosclerotic emboli in the renal arteries causing a renal infarct is primarily supportive.[64][65] The use of corticosteroids, anticoagulants, and statins has shown some benefits in limited studies, but the data is insufficient to recommend them as a routine measure.[1][64][66][67] However, if the patient developed the emboli while on anticoagulant therapy, it is recommended that anticoagulation be stopped based on the possibility that it may be contributing to plaque hemorrhage, which could induce or promote dislocation of plaque emboli.[68]
Additional angiographic and vascular procedures should be canceled, postponed, or delayed. Patients with renal infarcts due to atheroembolic have a relatively poor overall prognosis due to the severity of their underlying atherosclerotic disease. In one large study of 354 patients, 28% died within two years, and 33% developed end-stage kidney disease.[1] However, patients without significant comorbidities or complications can see their renal function improve over time which was shown in one study where 24% of the patients fully recovered.[21]
Other measures such as using aspirin, stopping smoking, improving hypertension control, improving weight management, and good glycemic control in patients with diabetes are generally recommended, but there is no supportive data for confirmation.
Summary
- CT renal angiography will establish the diagnosis and allow transcatheter treatment for acute cases, if appropriate.
- Thrombectomy is usually tried first, then thrombolysis.
- A continuous infusion of thrombolytics can be considered for up to 72 hours if bolus treatment is unsuccessful.
- Angioplasty and/or arterial stenting can be utilized when thrombolysis fails or is inappropriate.
- Systemic thrombolysis is reasonable in selected cases if catheter-directed therapy is not available.
- Systemic anticoagulation is recommended for late cases and is usually required for at least six months. Apixaban is suggested.
- Open surgery is appropriate for trauma cases and those associated with aortic dissections.
- Renovascular hypertension should be treated with ACE inhibitors.
Differential Diagnosis
The clinical presentation of renal infarction is similar to that of renal colic. Nephrolithiasis and pyelonephritis are the main differential diagnosis of renal infarction. Failure to consider renal infarction as a differential in a case of flank pain initially suspected as renal colic could delay diagnosis and make it unsalvageable with reperfusion therapy.[3]
Acute pyelonephritis can present with flank pain and fever. Urinalyses in pyelonephritis typically shows pyuria, bacteriuria, and WBC casts but generally do not help differentiate it from renal infarction.
Significant LDH elevation is not seen in pyelonephritis or nephrolithiasis and can be used to help distinguish these disorders from renal infarction, especially if serum transaminases are low.[8][17][35]
The radiological finding of perinephric stranding and edema is more indicative of pyelonephritis than renal infarction.[15] A contrast-enhanced CT scan of the abdomen may also help differentiate renal infarction from extrarenal causes of abdominal pain, including appendicitis, diverticulitis, ruptured aortic aneurysm, biliary colic, and gynecological diseases.[26]
Aortic dissection and renal cell carcinoma are other differentials for patients presenting with flank pain. Other causes of abdominal pain, like mesenteric ischemia, should also be ruled out because systemic embolization in a patient with atrial fibrillation could potentially occlude the blood vessels supplying the intestine leading to acute bowel infarction.
- Aortic aneurysm
- Aortic dissection
- Appendicitis
- Biliary colic
- Diverticulitis
- Gastroenteritis
- Gynecologic and ovarian disorders
- Mesenteric ischemia
- Nephrolithiasis
- "Passed" urinary stone
- Pancreatitis
- Pyelonephritis
- Renal cell carcinoma
Prognosis
In a retrospective study conducted on 44 patients with renal infarction and atrial fibrillation, Hazanov et al. found that the 30-day mortality in renal infarction was 11.4%.[42] In another study of 42 patients, there was a 17% reduction (10.4 cm to 8.54 cm) in the pole-to-pole length of the kidney in follow-up imaging of patients successfully treated with catheter-directed thrombolysis (9 out of 11 patients). In the same study, even though the kidneys showed adequate perfusion, there was a decrease in renal function in two patients (29% and 37% of the total renal function, respectively) demonstrated by a DMSA scan.[47] Bourgault et al. found impairment of renal function in 40.4% of cases of renal infarction.[4]
Yang et al. reported that 34.8% (31 of 89) of patients diagnosed with renal infarction also experienced acute kidney injury, and 27.1% developed chronic kidney disease during the follow-up period.[27] Most of these patients also had multiple comorbidities influencing morbidity and mortality.
The prognosis was found to be better in patients diagnosed early. Warm ischemia time, collateral flow, and preexisting kidney disease tend to influence prognosis following treatment with catheter-directed thrombolysis.[47]
Prognosis can also be influenced by embolic infarction in other organs like the spleen, intestine, liver, and lungs.[15] The presence of extrarenal embolisms increased the duration of hospital stay as well as the overall morbidity and mortality.[11]
Complications
In a single-center retrospective study conducted on 101 patients with renal infarction by Kwon et al. from 2005 to 2013, 41 (39%) patients developed acute kidney injury, and 27 out of 80 patients (33.8%) progressed to chronic kidney disease. In multivariate analysis, the risk factors for acute kidney injury in this study were hypertension and the presence of bilateral renal infarction.[69]
The incidence of acute kidney injury positively correlated with infarct size, the levels of AST, ALT, CRP, and LDH, the degree of proteinuria and hematuria, and WBC count in this study.
In another study by Yang et al, diabetes mellitus (p = 0.005), CRP (p = 0.001), and leukocytosis (p = 0.007) showed a significant relationship with acute kidney injury (P = 0.005).[27] In an observational study by Caravaca-Fontan et al., impaired kidney function was observed in 65% of diagnosed patients. There was an inverse and linear correlation between the CT scan assessment of ischemic damage and eGFR (R = -0.39, p = 0.001).[11]
The progression to chronic kidney disease from acute kidney injury correlated with the magnitude of the infarction.[70] The main risk factors for chronic kidney disease were advanced age and the degree of acute kidney injury. Acute kidney injury, even when fully recovered, was found to be a risk factor for the later development of chronic kidney disease, and 58% of renal infarction patients developed reduced renal function, as detected in follow-up renal scintigraphy.[70][71]
Consultations
In the case of acute renal infarction, it is advisable to seek consultation from a nephrologist.[3] Patients also require follow-up consultation with vascular surgery or interventional radiology if recurrent vascular lesions like fibromuscular dysplasia are discovered. This is because inflammation may prevent the accurate detection of fibromuscular dysplasia in the acute phase of renal infarction. One must also assess the carotid and other extra-renal arteries if fibromuscular dysplasia or other vasculopathies like Ehler-Danlos syndrome are suspected.[45] Also, cardiovascular and hematology consultations may be necessary to evaluate a recurrent event.
Deterrence and Patient Education
Patients should be educated regarding anticoagulation and the range of international normalized ratio (INR) maintenance. Those developing repeated infarcts, even with anticoagulant therapy, must be educated regarding medication compliance. Drug interactions and reactivity with certain food items must also be considered in the case of subtherapeutic INR in patients taking warfarin. If these considerations did not contribute to the recurrence of renal infarct, an adjustment in dosage or choosing another anticoagulant should be considered.
Pearls and Other Issues
A renal infarction should be suspected whenever a patient presents with acute flank pain and hematuria without evidence of a UTI. The initial non-contrast CT scan is negative for hydronephrosis or urinary calculi.
An immediate contrast-enhanced CT of the abdomen should be done as this is usually sufficient to make the diagnosis and can be done quickly. Otherwise, the diagnosis of a renal infarction is likely to be missed, along with the opportunity for effective revascularization.
A significantly elevated LDH is the only initial clinical or laboratory sign indicating a renal infarction at the time of initial presentation.
- Renal infarctions are frequently misdiagnosed on initial presentation.
- Patients with a history of atrial fibrillation, older age (especially with no prior history of nephrolithiasis), and recent vascular instrumentation are at higher risk.
- A CT angiogram is the definitive test to confirm the diagnosis.
- Laboratory work for patients diagnosed with a renal infarction should include a urinalysis, serum aminotransferases, creatinine, and LDH levels, as well as an EKG and coagulation studies.
- Rapid diagnosis allows for possible immediate catheter-directed thrombolysis therapy, which is the optimal recommended treatment if it can be initiated within 6 hours of the infarction.
- If catheter-directed thrombolysis therapy is unavailable, systemic thrombolytic therapy should be considered.
- Patients with renal infarction should be in regular follow-ups for the development of recurrent infarcts and chronic kidney injury or renal failure.
- Some patients may develop deterioration of renal function over time, eventually requiring hemodialysis.
- Follow-up CT or MR angiography may be needed six months after the acute event to assess the status of any vascular lesions leading to renal infarction and to ensure that no further infarcts have occurred.
- Patients may also require secondary prevention in the form of antiplatelet therapy, and most, if not all, should be on some permanent anticoagulation.
- In a patient with renal infarction, there has been controversy regarding the time until catheter-directed thrombolysis can be administered, but there are anecdotal reports of a benefit even after the usually recommended time limits.
- More prospective studies and randomized controlled trials are warranted to arrive at a clear guideline and consensus because the current literature is mostly retrospective studies and case reports.
Enhancing Healthcare Team Outcomes
The involvement of a multidisciplinary and interprofessional team involving clinicians (MDs and DOs, as well as mid-level practitioners such as NPs, and PAs), interventional radiologists, nephrologists, cardiologists, hematologists, and a vascular surgeon is required for the management of renal infarction. Nursing staff and pharmacists round out the interprofessional team.
While a clinician diagnoses the case of renal infarction, endovascular procedures are referred to an interventional radiologist or vascular surgeon. Complications such as acute kidney injury may need evaluation from a nephrologist. The etiology of renal infarction may require consultation with a cardiologist and hematologist. A combined approach by all these clinicians helps identify and tackle the rare challenging diagnosis of renal infarction.
Nursing will provide crucial support in patient examination and counseling, surgical preparation and assistance during surgery, and post-operative care. Pharmacists will be critical in all these cases, particularly with anticoagulation regimens, medication reconciliation, and counseling patients regarding their medication.
Any multidisciplinary/interprofessional healthcare team member who notes a change in patient status performs an intervention themselves or notes therapeutic failure or an adverse event should immediately record their findings in the patient's chart or medical record and notify other team members so that corrective action can be implemented. This kind of interprofessional information sharing and collaborative action will optimize patient outcomes in renal infarction cases and prevent any adverse results. [Level 5]