Equipment
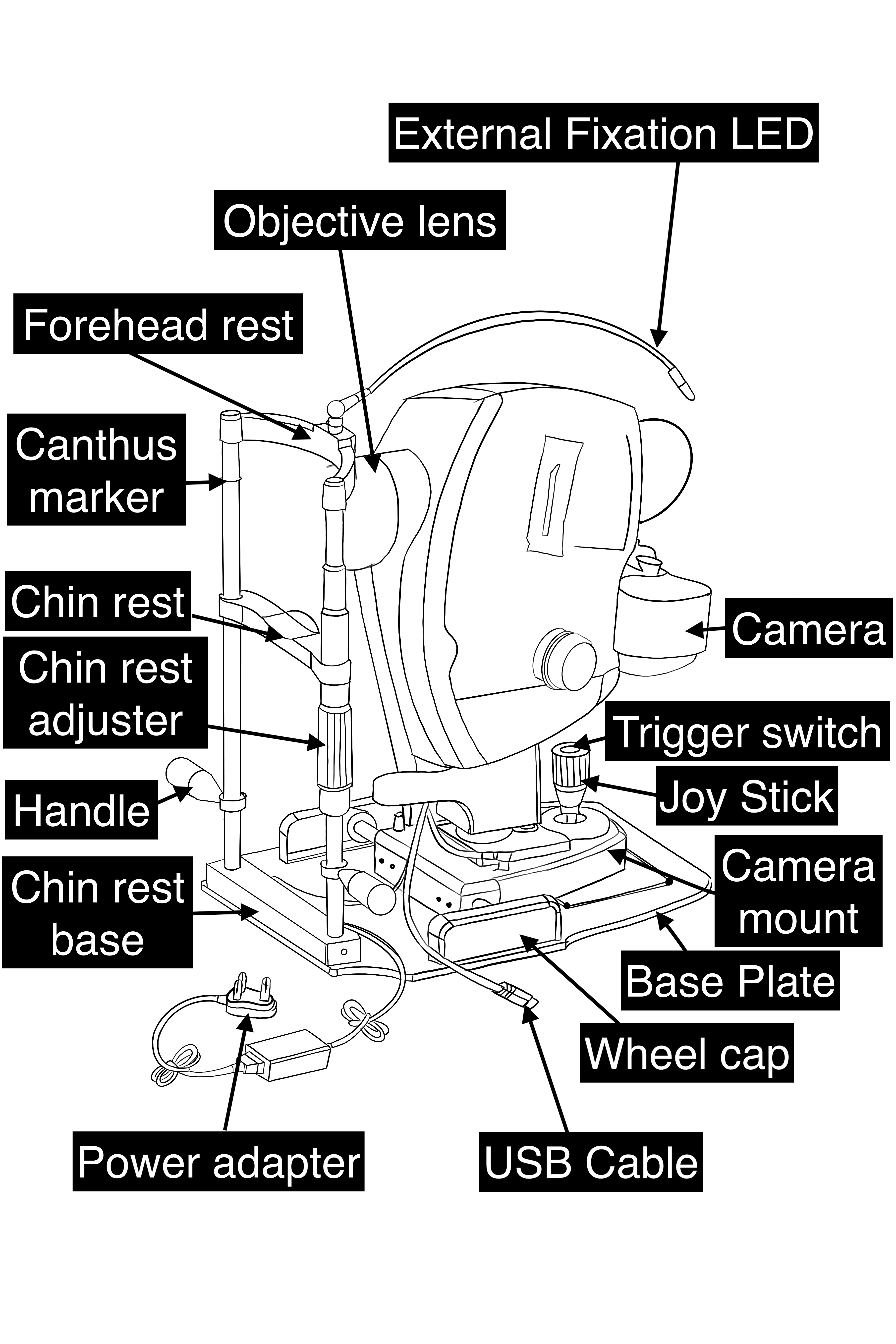
A fundus camera is a complex system with multiple lenses and a camera utilizing the principle of indirect ophthalmoscope (Figure-2). The parts of a typical fundus camera are shown in the figure (Figure-3).
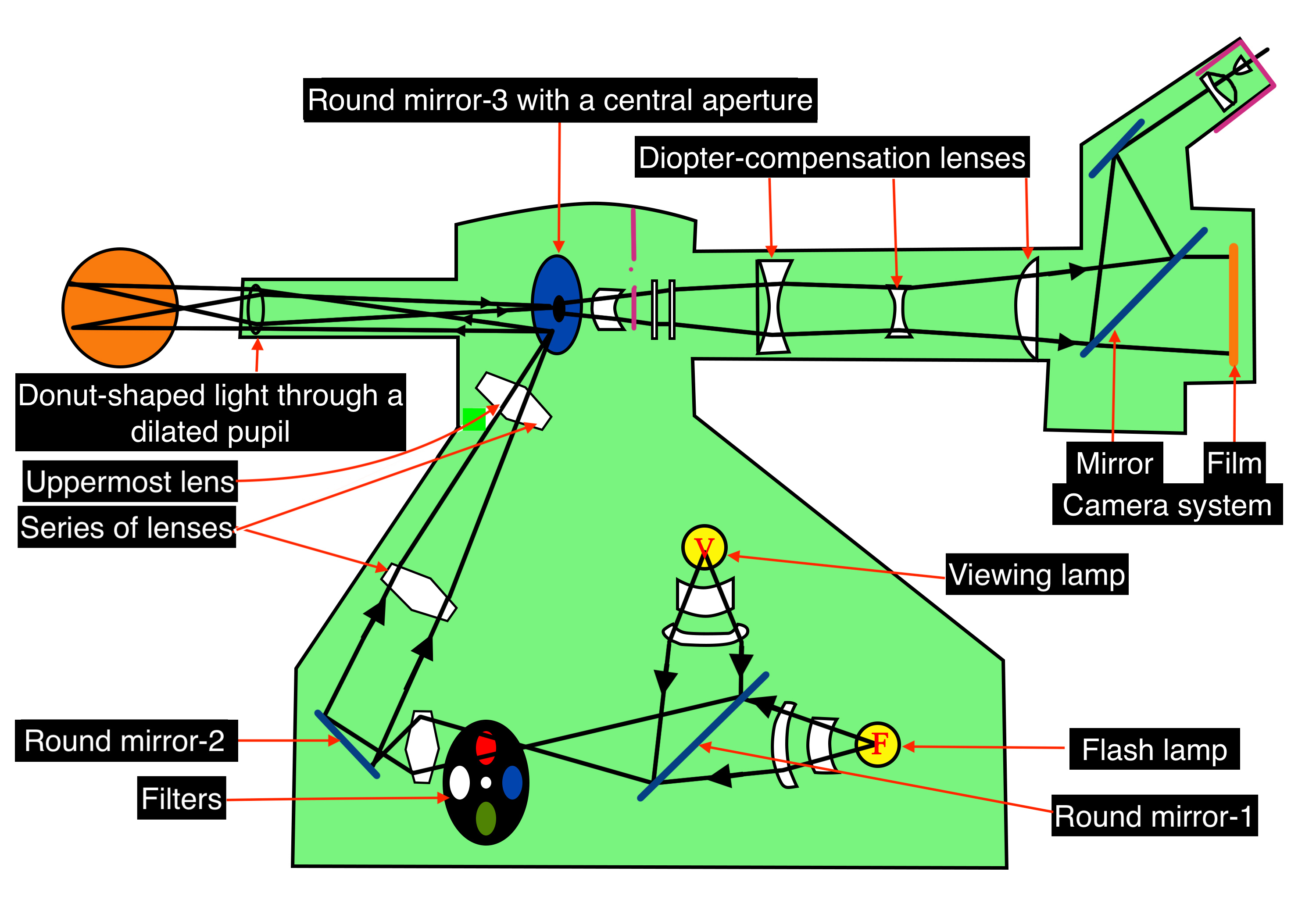
Optics of Fundus Camera
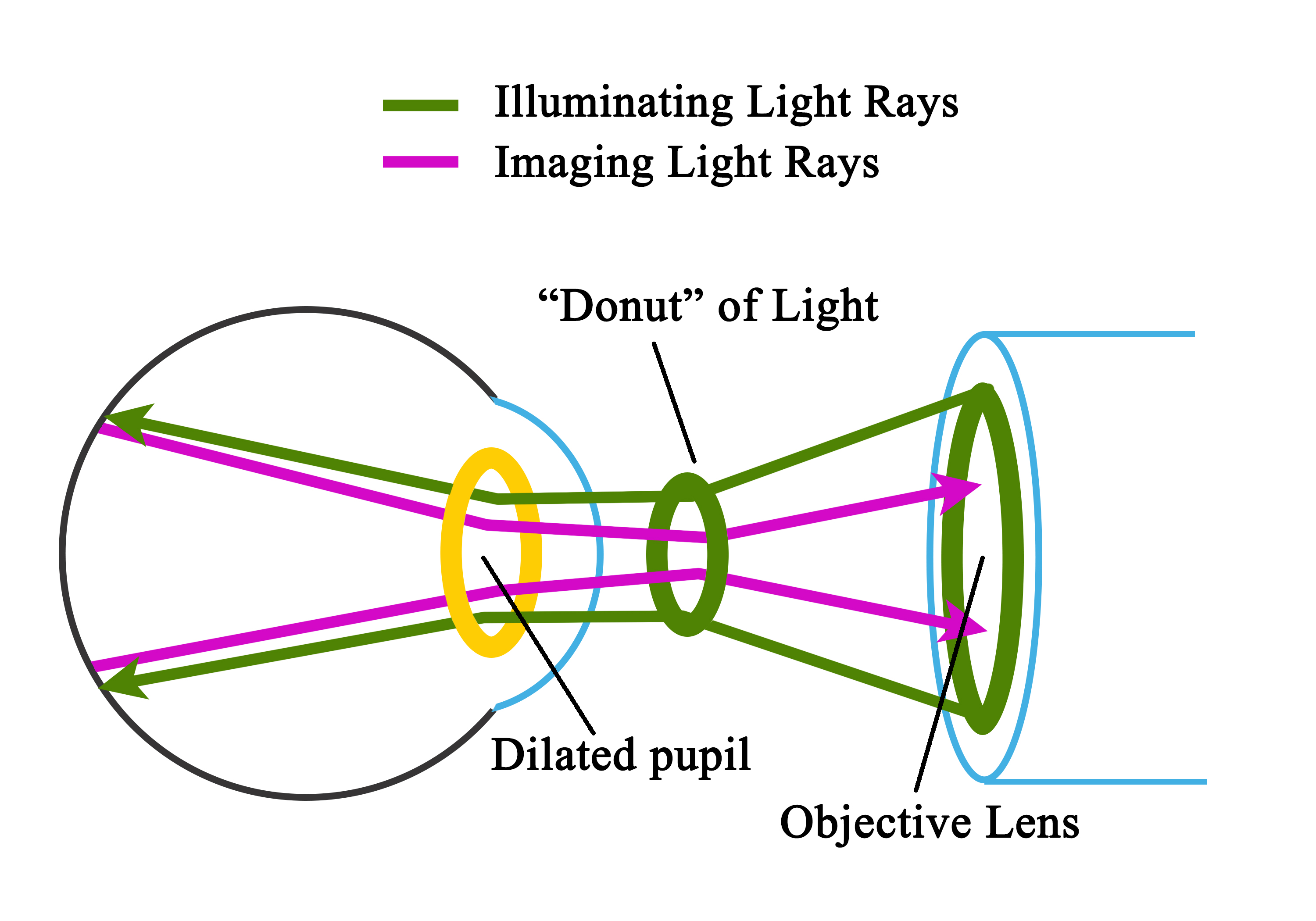
Light from the viewing lamp is projected onto a round mirror (Figure-4, round mirror-1) and then through a set of filters (Figure-4, filters) onto another round mirror (Figure 4, round mirror -2). The light is reflected from the mirror (round mirror-2) to a series of lenses (Figure 4, series of lenses).
Due to the presence of a mask in the uppermost lens (Figure 4, uppermost lens), the light is finally focused as a donut-shaped beam. This donut-shaped light beam falls on another round mirror with a central aperture (Figure-4, round mirror-3 with a central aperture), and the reflection exits the camera system through the objective lens and enters through the cornea into the eye (Figure-4, donut-shaped light through a dilated pupil), the photo of which is to be taken.
When both the illuminating rays through the pupillary border and the image at the center of the pupil are correctly aligned, the imaging rays exit through the center of the pupil, i.e., the central non-illuminated portion of the donut of the light (Figure-1, Figure-4, donut-shaped light through a dilated pupil)
The imaging rays pass through the central aperture of the mirror as mentioned above (Figure-4, round mirror-3 with a central aperture), through the astigmatic correction and diopter compensation lenses (Figure-4, diopter compensation lenses), then back to the single-lens reflex camera system (Figure-4, camera system).
The commonly used table-top fundus cameras include iCam (Optovue Inc. Fremont, CA), 3nethra (Forus Inc. Bengaluru, India), digital retinography system (dRS, CenterVue, Padova, Italy), EasyScan (iOptics, Den Haag, The Netherlands), TRCNW8Fplus (Topcon, Tokyo, Japan), Visucam (Zeiss, Oberkochen, Germany), Nonmyd7 (Kowa, Torrance, CA), and Canon CR-2 ( Canon, Tokyo, Japan).[1]
Over time, there have been many upgrades to the traditional table-top fundus camera. These are as described below.
Wide-field and ultra-widefield fundus cameras:
Wide-field fundus imaging refers to taking retinal images of more than 50 degrees.[25] Ultra-wide-field fundus imaging was defined by DRCR.net (diabetic retinopathy clinical research network) to be more than 100 degrees of field of area.[26]
Recently a consensus nomenclature defined wide-field imaging (WFI) to be a single captured retinal image centered on the fovea and extending beyond the posterior pole but posterior to the vortex vein ampullae in all the four quadrants. Ultra-wide-field image (UWFI) is a single captured retinal image that captures retinal images anterior to the vortex vein ampullae in all four quadrants.[27]
|
WFI and UWFI Systems
|
Contact/ Non-contact
|
Field of area
|
Method of imaging
|
Advantages
|
Disadvantages
|
|
Equator-plus camera [by O Pomerantzeff][28]
|
Contact
|
148 degrees from the nodal point
|
Patient in sitting position
|
One of the earlier introduced UWFI fundus cameras is equipped with interference filters.
|
The peripheral illumination through the peripheral cornea reduces high resolution and causes brilliance/bright artifacts at the peripheral retina.
|
|
RetCam (Clarity Medical Systems, Inc., Pleasanton, CA, USA).
|
Contact
|
130- degree
|
The patient is in the supine position. It is mainly used for retinopathy of prematurity (ROP) babies.
|
Contact ultra-widefield fundus camera for a neonatal eye examination. The machine can be transported to neonatal intensive care units along with the ROP team. The images can be sent to the base hospital via the internet/cloud.
|
High cost of the machine. The learning curve for the personnel capturing the images.
|
|
Optos® camera (Optos PLC, Dunfermline, UK)
|
Non-contact
|
Up to 200 degrees
|
Sitting position or flying baby position
|
A non-contact ultra-widefield fundus camera has the widest field of view, i.e., up to 200 degrees. UWF Fundus auto-fluorescence (FAF) can be done. Upgraded versions have features like UWF fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), and UWF optical coherence tomography (OCT).[23][15][29][30]
|
High cost of the machine.
cSLO images are not true color fundus images.
|
|
Heidelberg Spectralis with the Staurenghi lens (Ocular Staurenghi 230 SLO Retina Lens; Ocular Instruments Inc, Bellevue, WA, USA)
|
Non-contact and Contact
|
105 degrees in non-contact [31]
150 degrees in contact Staurenghi lens
|
The sitting position of the patient
|
Both non-contact and contact fundus imaging modes are available.
|
High cost of the machine.
cSLO images are not true color fundus images.
|
|
Clarus® 500 (Carl Zeiss Meditec)
|
Non-Contact
|
Single capture 133 degrees, Montage 200 degrees
|
The sitting position of the patient
|
Non-contact ultra-widefield True color imaging, auto-fluorescence, Blue, Green, Infrared, and FFA can be done.
|
High cost of the machine.
|
|
Mirante device by Nidek (Nidek Co., Ltd, Gamogori, Japan)
|
Non-Contact
|
163° ultra-wide field imaging with a single image capture
|
The sitting position of the patient
|
Color fundus photo, FFA, ICG, FAF, retro mode imaging, OCT, angio-scan OCT-angiography, and anterior segment OCT.
|
High cost of the machine. cSLO images are not true color fundus images.
|
|
Eidon (CenterVue, Italy)
|
Non-contact
|
Single capture 90 degrees maximum (measured from the center of the eye), up to 160 degrees in a mosaic fashion.
|
The sitting position of the patient
|
True color fundus images with advantages of cSLO images. Infra-red and red-free images are available.
|
The field of view in a single capture is 90 degrees maximum, which is not UWF imaging
|
The Optos non-contact ultra-widefield pseudocolor fundus camera uses a scanning laser ophthalmoscope (SLO), two laser light sources of wavelengths 532 nm (green) and either 633 nm or 635 nm (red), and an ellipsoidal mirror with two conjugal focal points which enables capturing the retinal images up to 200 degrees.[32]
Advantages of WFI and UWFI
- Better resolution
- Possible for image acquisition in non-compliant young pediatric patients
- Patients with undilated pupils and hazy media due to cataracts or other diseases
- Simultaneous imaging of central and peripheral retina; use of UWF FFA to evaluate peripheral retinal ischemia and new vessels in patients with familial exudative vitreoretinopathy, diabetic macular edema, and other vascular complications.
- Counseling and education of the patients
Confocal scanning laser ophthalmoscope (cSLO) based fundus cameras: The confocal scanning laser ophthalmoscope (cSLO) uses laser light instead of a flashlight to illuminate the retina. This reduces the scatter of light in the images acquired, forming a sharp, high-contrast image located within the focal plane.
Currently, the imaging systems using cSLO-based fundus imaging are increasingly used. The common cSLO-based fundus cameras include Optos, the non-contact ultra-widefield fundus camera with 200 degrees field of view. This imaging system has UWF fundus imaging, choroidal imaging, fundus autofluorescence (FAF), fundus fluorescein angiography (FFA), indocyanine green (ICG) angiography, and optical coherence tomography (OCT) imaging.[33]
Heidelberg Spectralis multi-color imaging has additional features like OCT, OCT-angiography, and fundus auto-fluorescence (FAF).
Using Mirante, the cSLO-based multimodal imaging platform, in addition to the UWF fundus photo, the additional features like FFA, ICGA, FAF, and retro mode imaging for visualizing pathologies deeper than the retinal pigment epithelium (RPE), and detecting pathologic changes in the choroid, OCT, OCT-Angiography, and an anterior segment OCT with an additional adapter can be performed.
Eidon (CenterVue) combines the cSLO with the conventional fundus imaging (white light emitting diode/LED) system to give true color-wide field fundus images.
Red-free fundus photography (RFFP): The red-free fundus photography (RFFP) is monochromatic retinal imaging that uses gree contrast filters and enables an excellent view of the retina as the peak spectral sensitivity of the human eye falls in the green-yellow range of the spectrum. Retinal vasculature and nerve fiber layers are better seen in RFFP. The RFFP image is routinely taken as a baseline image before the FFA.[34]
Fundus autofluorescence (FAF): The FAF uses the fluorescent properties of the fluorophores. The important fluorophores in the retina are lipofuscin and melanin. Lipofuscin is a byproduct of the lysosomal breakdown of the outer photoreceptor segments within the RPE. The concentration of the lipofuscin pigments increases from the equator of the retina to the posterior pole, except for the fovea.
Lipofuscin absorbs light in the blue spectrum with a wavelength of 470 nm and emits the light in the yellow-green spectrum at a wavelength of 600 to 610 nm. Lipofuscin-based autofluorescence is known as shortwave autofluorescence (SWAF) or blue auto-fluorescence (BAF). Melanin has an excitation wavelength of 787 nm and an emission wavelength at a near-infrared wavelength. Melanin-based autofluorescence is known as near-infrared autofluorescence (NIRAF).[35]
In BAF, the fovea is darker (hypo-autofluorescent) due to less lipofuscin at the fovea. In contrast, in NIRAF, the fovea is brighter (hyper-autofluorescent) due to the highest concentration of melanin in this region. FAF has diagnostic and prognostic utility in various diseases like central serous chorioretinopathy (CSCR), age-related macular degeneration (ARMD), retinal dystrophies, and uveitic entities like choroiditis. FAF images of the retina can be taken in a conventional fundus camera with a 50 to 55-degree field of view with the facility of FAF. UWF FAF is available in Optos, Heidelberg Spectralis, Mirante, and Clarus.
Multi-color imaging: Multi-color imaging (MCI) uses cSLO to capture three reflectance images simultaneously 1) blue reflectance (BR; 488 nm), (2) green reflectance (GR; 515 nm), and the (3) near-infrared reflectance (IR; 820 nm). The blue reflectance enables the details of the inner retina, the green reflectance details the retinal blood vessels and exudations in the retinal layers, if any, and the infrared reflectance details the outer retina, including the RPE abnormalities. The near-infrared reflectance has been most helpful in imaging the choroidal nodules in neurofibromatosis type 1, and these nodules have been correlated with the presence of Lisch nodules of the iris.[36]
The blue reflectance is better than the green reflectance for detecting dissociated optic nerve fiber layer after internal limiting membrane peeling.[37] Multi-color images are captured at both 30 degrees and 55 degrees in the Heidelberg Spectralis machine. In the Mirante imaging system, 163 degrees UWF multi-color images with single image capture are possible. MCI has been beneficial in diagnosing and managing diseases like ARMD, diabetic retinopathy, vitreoretinal interface disorders, retinal vein occlusions, and retinal dystrophies.[38]
Multi-color imaging is useful in detecting vitreoretinal interface abnormalities better than posterior uveitic lesions like retinochoroiditis and retinal lesions like intraretinal hemorrhages.[39]
Smartphone-Based fundus cameras: Recently, smartphone-based fundus imaging has been proven to be a low-cost alternative to standard fundus photography. It can capture images in both the dilated and undilated pupils. It has an immense role in screening diseases like DR, ARMD, ROP, and Glaucoma. It is becoming a boon for teleophthalmology.[40] Low-cost fundus cameras have been developed to help screen for retinal diseases in remote parts of the world.[41]
Smartphone-based fundus photography has been effective in detecting DR.[42] In smartphone-based fundus photography, the diagnostic accuracy for PDR is 92%, and that for referable DR is 91%.[43]
Some of the FDA-approved smartphone-based fundus imaging systems include Welch Allyn iExaminer (Welch Allyn, Skaneateles Falls, NY), Remidio Fundus on Phone device (Remidio Innovative Solutions, Bangalore, India), D-Eye, and iNview (Volk Optical, Inc.). Recently, the integration of artificial intelligence (AI) has increased the capability of fundus imaging, especially in the screening of DR.[44]
IDx-DR is an autonomous AI system that has been approved by the USFDA for the detection of DR stages more than mild DR or absence of DR. It has an imageability rate of 96.1%, a sensitivity of 87.2%, and a specificity of 90.7%.[45] USFDA cleared EyeArt (Eyenuk) for autonomous detection of more than mild DR and vision-threatening DR.[4]
Technique or Treatment
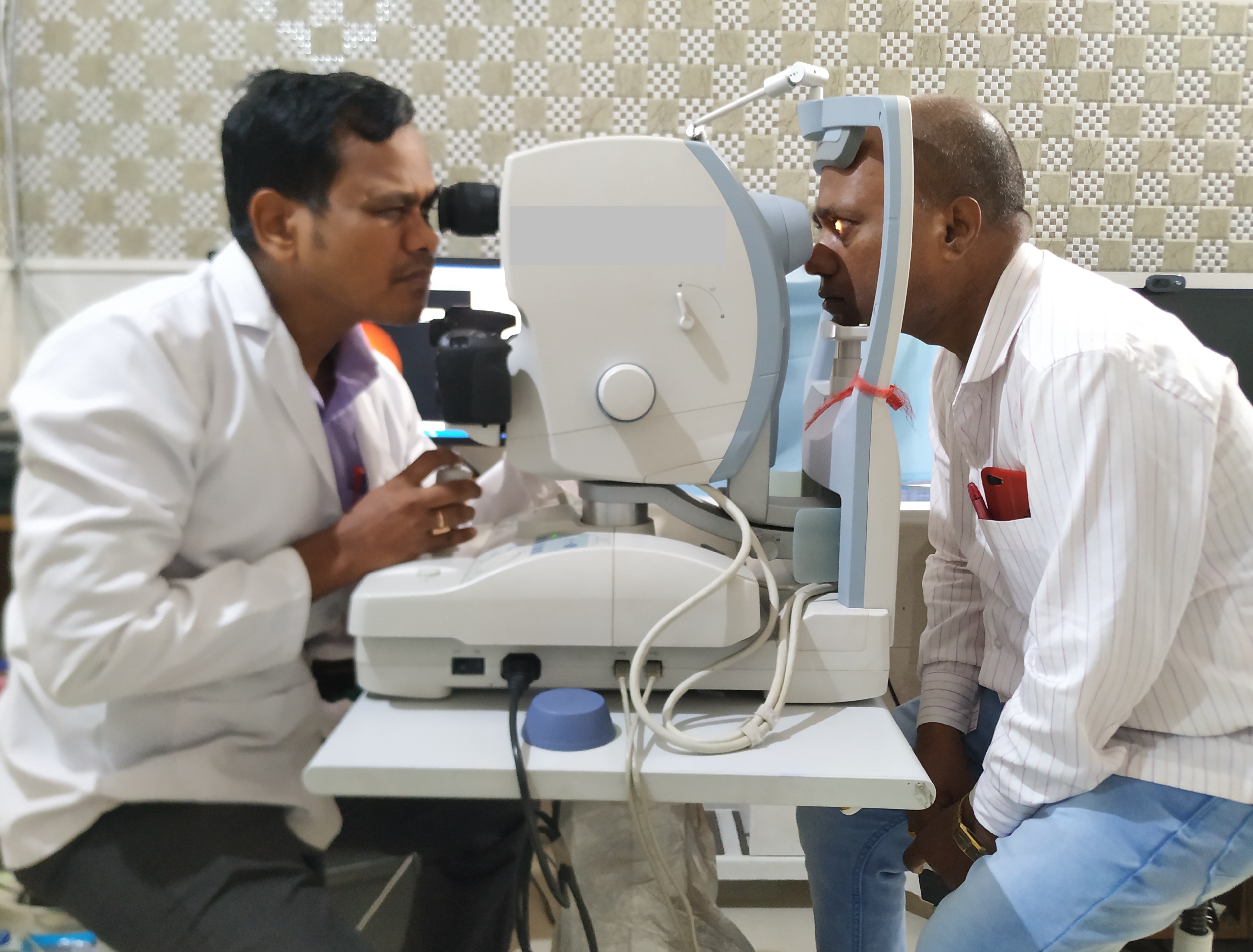
A photo of one eye of the patient is taken at a time. First, align the circle on the screen with the patient’s iris and pupil. Adjust the height to make the three white dots in a symmetric position. Then focus on the retina of the patient. Ask the patient to focus on the green light they see. Adjust the joystick forwards, backward, and sideways to get a sharper view of the two dots on the three o clock and nine o clock positions (to ensure the optimal distance of the camera from the eye) or a sharper image of the reticules.
There is an indicator indicating the good focus of the retina (two vertical lines which have to be aligned in some cameras). For manual capture, the eyepiece must be correctly set. The photographer must wear the refractive error corrections, if any. The accommodation of the photographer must be at rest. When the reticules are sharper, and the view of the retina is sharper, the button is pressed to capture the image (Figure-5). Fundoscopy has been taught to students using virtual platforms, which proved useful, especially during the COVID pandemic.[46]
Stereophotography uses two images taken from the right and left, which are then viewed with a stereo viewer. Optional lenses must be employed for focusing anterior objects (mid-vitreous, anterior segment). To focus posterior objects or the retina in pathological myopia, minus lenses have to be added. For extremes of refractive errors (around +15 diopter or -15 diopters), the autofocus function of the fundus camera may not work, and manual focusing should be used to focus the retina.
Clinical Significance
Fundus cameras are used for fundus photography. Fundus photography is useful for diagnosing, educating patients, counseling, monitoring, and forecasting many ophthalmic conditions, notably DR, ARMD, Retinal vascular disorders, ROP, and glaucoma (Figures).
The traditional tabletop fundus cameras with a 50-degree field of view can detect the pathology of the posterior pole of the eye. The wide field and ultra-widefield fundus cameras are helpful in the imaging of retinal pathology beyond the equator of the retina. The presence of predominantly peripheral lesions in DR can be detected with the help of UWFI and carries a higher risk of progression to PDR.[47]
The contact ultra-widefield fundus camera for a neonatal eye examination (RetCam) has been used extensively for the diagnosis and follow-up of neonates at risk with and suffering from retinopathy of prematurity (ROP). This camera is portable and can be taken to remote places.
Advances in Fundus Imaging and Future Perspectives
Bog data, machine learning, and artificial intelligence are promising in improving healthcare.[48] The integration of AI into the fundus cameras has shown good accuracy for the detection of diseases like DR. Machine learning algorithms using preoperative fundus photography along with some other preoperative data, including anterior chamber depth, central corneal depth, age, planned ablation thickness, has been shown to identify at-risk eyes for postoperative myopic regression after refractive surgery.[49]
Deep learning algorithms using fundus photographs have been used to predict cerebral white matter hyperintensity in magnetic resonance imaging (MRI) scans.[50] A deep learning framework for the earlier detection of DR from fundus images has been developed, and it could differentiate the healthy and harmful images with 95.6% precision.[51] Studies using AI to establish the role of fundus photos, OCT, and external eye photography with systemic diseases and parameters show promising results.[52]
AI for the screening of DR has shown a lot of promise. These findings underscore the prospective role of AI integrated fundus imaging in the screening, diagnosis, and management of different retinal diseases.[53]
Combining OCT imaging with fundus photography has been shown to increase the efficacy of glaucoma screening.[54] This combination has also been shown to effectively diagnose cases of high myopia with retinopathy.[55] This approach demonstrates the beneficial role of multi-modal imaging. Studies on handheld fundus cameras are on the rise. Comparative studies have shown that the handheld fundus camera effectively diagnoses referrable cases of DR.[56]
Similarly, smartphone-based fundus imaging is on the rise and is most useful for teleophthalmology. However, in detecting ROP, though the smartphone-based cameras provide a moderate agreement for the detection of the presence or absence of plus disease, they failed to identify the stages and zones of the disease.[57]