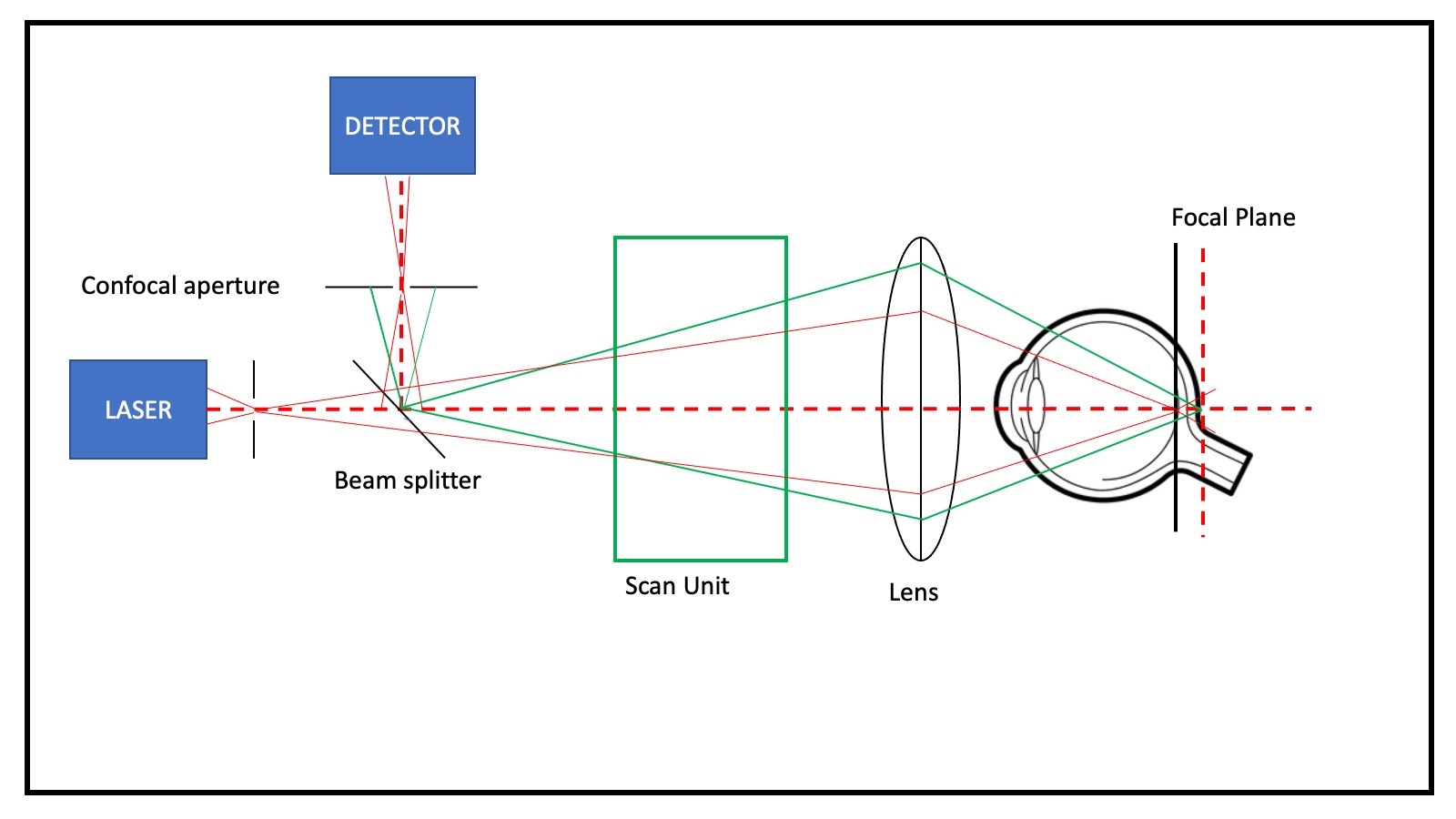
[1]
Mainster MA,Desmettre T,Querques G,Turner PL,Ledesma-Gil G, Scanning laser ophthalmoscopy retroillumination: applications and illusions. International journal of retina and vitreous. 2022 Sep 30;
[PubMed PMID: 36180893]
[2]
Fischer J,Otto T,Delori F,Pace L,Staurenghi G,Bille JF, Scanning Laser Ophthalmoscopy (SLO) High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. 2019;
[PubMed PMID: 32091845]
[3]
Gupta K,Agarwal A,Arora A,Aggarwal K,Bansal R,Katoch D,Marchese A,Singh SR,Agrawal R,Gupta V, MULTICOLOR CONFOCAL SCANNING LASER OPHTHALMOSCOPE IMAGING IN POSTERIOR UVEITIS. Retina (Philadelphia, Pa.). 2022 Jul 1;
[PubMed PMID: 35723923]
[4]
Vienola KV,Lejoyeux R,Gofas-Salas E,Snyder VC,Zhang M,Dansingani KK,Sahel JA,Chhablani J,Rossi EA, Autofluorescent hyperreflective foci on infrared autofluorescence adaptive optics ophthalmoscopy in central serous chorioretinopathy. American journal of ophthalmology case reports. 2022 Dec;
[PubMed PMID: 36345414]
Level 3 (low-level) evidence
[5]
Xu Q,Rydz C,Nguyen Huu VA,Rocha L,Palomino La Torre C,Lee I,Cho W,Jabari M,Donello J,Lyon DC,Brooke RT,Horvath S,Weinreb RN,Ju WK,Foik A,Skowronska-Krawczyk D, Stress induced aging in mouse eye. Aging cell. 2022 Nov 17;
[PubMed PMID: 36397653]
[6]
Curcio CA,Johnson M,Huang JD,Rudolf M, Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. Journal of lipid research. 2010 Mar
[PubMed PMID: 19797256]
[7]
Michalinos A, Zogana S, Kotsiomitis E, Mazarakis A, Troupis T. Anatomy of the Ophthalmic Artery: A Review concerning Its Modern Surgical and Clinical Applications. Anatomy research international. 2015:2015():591961. doi: 10.1155/2015/591961. Epub 2015 Nov 9
[PubMed PMID: 26635976]
[9]
Littlewood R,Mollan SP,Pepper IM,Hickman SJ, The Utility of Fundus Fluorescein Angiography in Neuro-Ophthalmology. Neuro-ophthalmology (Aeolus Press). 2019 Aug
[PubMed PMID: 31528186]
[10]
Hayreh SS, Segmental nature of the choroidal vasculature. The British journal of ophthalmology. 1975 Nov;
[PubMed PMID: 812547]
[13]
Albrecht May C, Comparative anatomy of the optic nerve head and inner retina in non-primate animal models used for glaucoma research. The open ophthalmology journal. 2008 May 9;
[PubMed PMID: 19516911]
Level 2 (mid-level) evidence
[14]
Cameron JR,Megaw RD,Tatham AJ,McGrory S,MacGillivray TJ,Doubal FN,Wardlaw JM,Trucco E,Chandran S,Dhillon B, Lateral thinking - Interocular symmetry and asymmetry in neurovascular patterning, in health and disease. Progress in retinal and eye research. 2017 Jul;
[PubMed PMID: 28457789]
[15]
Tan AC,Fleckenstein M,Schmitz-Valckenberg S,Holz FG, Clinical Application of Multicolor Imaging Technology. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2016;
[PubMed PMID: 27404384]
[16]
Schmitz-Valckenberg S,Pfau M,Fleckenstein M,Staurenghi G,Sparrow JR,Bindewald-Wittich A,Spaide RF,Wolf S,Sadda SR,Holz FG, Fundus autofluorescence imaging. Progress in retinal and eye research. 2021 Mar;
[PubMed PMID: 32758681]
[17]
Jiang Z,Yu Z,Feng S,Huang Z,Peng Y,Guo J,Ren Q,Lu Y, A super-resolution method-based pipeline for fundus fluorescein angiography imaging. Biomedical engineering online. 2018 Sep 19;
[PubMed PMID: 30231879]
[18]
Invernizzi A,Pellegrini M,Cornish E,Yi Chong Teo K,Cereda M,Chabblani J, Imaging the Choroid: From Indocyanine Green Angiography to Optical Coherence Tomography Angiography. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2020 Jul-Aug;
[PubMed PMID: 32739938]
[19]
Battu R,Dabir S,Khanna A,Kumar AK,Sinha Roy A, Adaptive optics imaging of the retina. Indian journal of ophthalmology. 2014 Jan;
[PubMed PMID: 24492503]
[20]
Alexandrescu C,Dascalu AM,Panca A,Sescioreanu A,Mitulescu C,Ciuluvica R,Voinea L,Celea C, Confocal scanning laser ophthalmoscopy in glaucoma diagnosis and management. Journal of medicine and life. 2010 Jul-Sep;
[PubMed PMID: 20945812]
[21]
Le Gargasson JF,Paques M,Guez JE,Boval B,Vicaut E,Hou X,Grall Y,Gaudric A, Scanning laser ophthalmoscope imaging of fluorescein-labelled blood cells. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1997 Jan
[PubMed PMID: 9034843]
[22]
Meira J,Marques ML,Falcão-Reis F,Rebelo Gomes E,Carneiro Â, Immediate Reactions to Fluorescein and Indocyanine Green in Retinal Angiography: Review of Literature and Proposal for Patient's Evaluation. Clinical ophthalmology (Auckland, N.Z.). 2020
[PubMed PMID: 32021082]
[23]
Holz FG,Bellmann C,Rohrschneider K,Burk RO,Völcker HE, Simultaneous confocal scanning laser fluorescein and indocyanine green angiography. American journal of ophthalmology. 1998 Feb;
[PubMed PMID: 9467450]
[24]
Yang Q,Miao Y,Huo T,Li Y,Heidari E,Zhu J,Chen Z, Deep imaging in highly scattering media by combining reflection matrix measurement with Bessel-like beam based optical coherence tomography. Applied physics letters. 2018 Jul 2
[PubMed PMID: 30034015]
Level 3 (low-level) evidence
[25]
Staurenghi G,Viola F,Mainster MA,Graham RD,Harrington PG, Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Archives of ophthalmology (Chicago, Ill. : 1960). 2005 Feb
[PubMed PMID: 15710823]
[26]
Sharp PF,Manivannan A,Xu H,Forrester JV, The scanning laser ophthalmoscope--a review of its role in bioscience and medicine. Physics in medicine and biology. 2004 Apr 7;
[PubMed PMID: 15128191]
[27]
Barteselli G,Chhablani J,Lee SN,Wang H,El Emam S,Kozak I,Cheng L,Bartsch DU,Azen S,Freeman WR, Safety and efficacy of oral fluorescein angiography in detecting macular edema in comparison with spectral-domain optical coherence tomography. Retina (Philadelphia, Pa.). 2013 Sep
[PubMed PMID: 23584697]
[28]
Gupta V,Rajendran A,Narayanan R,Chawla S,Kumar A,Palanivelu MS,Muralidhar NS,Jayadev C,Pappuru R,Khatri M,Agarwal M,Aurora A,Bhende P,Bhende M,Bawankule P,Rishi P,Vinekar A,Trehan HS,Biswas J,Agarwal R,Natarajan S,Verma L,Ramasamy K,Giridhar A,Rishi E,Talwar D,Pathangey A,Azad R,Honavar SG, Evolving consensus on managing vitreo-retina and uvea practice in post-COVID-19 pandemic era. Indian journal of ophthalmology. 2020 Jun
[PubMed PMID: 32461407]
Level 3 (low-level) evidence
[29]
Nettrour JF,Burch MB,Bal BS, Patients, pictures, and privacy: managing clinical photographs in the smartphone era. Arthroplasty today. 2019 Mar
[PubMed PMID: 31020023]
[30]
Aceto P,Antonelli Incalzi R,Bettelli G,Carron M,Chiumiento F,Corcione A,Crucitti A,Maggi S,Montorsi M,Pace MC,Petrini F,Tommasino C,Trabucchi M,Volpato S,Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI), Società Italiana di Gerontologia e Geriatria (SIGG), Società Italiana di Chirurgia (SIC), Società Italiana di Chirurgia Geriatrica (SICG) and Associazione Italiana di Psicogeriatria (AIP), Perioperative Management of Elderly patients (PriME): recommendations from an Italian intersociety consensus. Aging clinical and experimental research. 2020 Sep
[PubMed PMID: 32651902]
Level 3 (low-level) evidence
[31]
Jacquet GA, Hamade B, Diab KA, Sawaya R, Dagher GA, Hitti E, Bayram JD. The Emergency Department Crash Cart: A systematic review and suggested contents. World journal of emergency medicine. 2018:9(2):93-98. doi: 10.5847/wjem.j.1920-8642.2018.02.002. Epub
[PubMed PMID: 29576820]
Level 1 (high-level) evidence
[32]
Attiku Y,He Y,Nittala MG,Sadda SR, Current status and future possibilities of retinal imaging in diabetic retinopathy care applicable to low- and medium-income countries. Indian journal of ophthalmology. 2021 Nov
[PubMed PMID: 34708731]
[34]
Gadde SGK,Sridharan A,Gurram NR,Poornachandra B,Govindasamy N,Jayadev C, Merits of multicolor imaging for tractional retinal detachment. Indian journal of ophthalmology. 2022 Feb
[PubMed PMID: 35086217]
[35]
Sun Y,Smith LEH, Retinal Vasculature in Development and Diseases. Annual review of vision science. 2018 Sep 15
[PubMed PMID: 30222533]
[36]
Abdolrahimzadeh S, Ciancimino C, Grassi F, Sordi E, Fragiotta S, Scuderi G. Near-Infrared Reflectance Imaging in Retinal Diseases Affecting Young Patients. Journal of ophthalmology. 2021:2021():5581851. doi: 10.1155/2021/5581851. Epub 2021 Jul 31
[PubMed PMID: 34373789]
[37]
Spooner K,Phan L,Cozzi M,Hong T,Staurenghi G,Chu E,Chang AA, Comparison between two multimodal imaging platforms: Nidek Mirante and Heidelberg Spectralis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2021 Jul;
[PubMed PMID: 33409677]
[38]
Sparrow JR,Wu Y,Nagasaki T,Yoon KD,Yamamoto K,Zhou J, Fundus autofluorescence and the bisretinoids of retina. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2010 Nov
[PubMed PMID: 20862444]
[39]
Cideciyan AV,Swider M,Aleman TS,Roman MI,Sumaroka A,Schwartz SB,Stone EM,Jacobson SG, Reduced-illuminance autofluorescence imaging in ABCA4-associated retinal degenerations. Journal of the Optical Society of America. A, Optics, image science, and vision. 2007 May
[PubMed PMID: 17429493]
[40]
Tsang SH,Sharma T, Fundus Autofluorescence. Advances in experimental medicine and biology. 2018;
[PubMed PMID: 30578477]
Level 3 (low-level) evidence
[43]
Lu J,Gu B,Wang X,Zhang Y, High-speed adaptive optics line scan confocal retinal imaging for human eye. PloS one. 2017;
[PubMed PMID: 28257458]
[44]
Patel CK,Buckle M, Ultra-Widefield Imaging for Pediatric Retinal Disease. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2018 May-Jun;
[PubMed PMID: 29888558]
[46]
Woetzel AK,Lauermann JL,Kreitz K,Alnawaiseh M,Clemens CR,Eter N,Alten F, Optical coherence tomography angiography image quality assessment at varying retinal expertise levels. Journal of current ophthalmology. 2019 Jun
[PubMed PMID: 31317094]
Level 2 (mid-level) evidence