Continuing Education Activity
Corneal pathologies are significant contributors to global blindness rates. Visual impairment often stems from conditions such as corneal opacity, keratoconus, corneal hydrops, pellucid marginal degeneration, spheroidal degeneration, pseudophakic bullous keratopathy, Fuchs endothelial dystrophy, corneal dystrophy, and previously failed graft. In 1905, Eduard Konrad Zirm achieved a significant milestone by performing the world's first full-thickness solid organ corneal transplant. Optical penetrating keratoplasty (OPK) or penetrating keratoplasty (PKP) is a surgical procedure involving the complete replacement of the host cornea with a full-thickness donor corneal graft. The use of an appropriate-sized trephine facilitates the removal of the recipient cornea.
When performed with diligent postoperative care, corneal transplants can yield successful outcomes lasting for decades. However, OPK carries potential intraoperative and postoperative complications that require timely management to achieve optimal results. Graft rejection episodes can occur at any time following optical keratoplasty, and multiple risk factors contribute to the likelihood of rejection. This activity aims to provide focused discussions on the anatomy and physiology of the cornea, indications and contraindications for OPK, necessary equipment, surgical technique, complications, and the clinical significance of optical penetrating keratoplasty.
Objectives:
- Differentiate between various corneal conditions that may require penetrating keratoplasty versus alternative treatment options.
- Implement proper surgical techniques and protocols during the procedure to ensure optimal outcomes for the patient.
- Assess the patient's preoperative condition, including visual acuity, corneal measurements, and overall ocular health, to determine the appropriateness of penetrating keratoplasty.
- Collaborate with other healthcare professionals, such as ophthalmologists, optometrists, nurses, and counselors, to provide comprehensive and coordinated care throughout the patient's journey.
Introduction
Penetrating keratoplasty (PKP) or optical penetrating keratoplasty (OPK) is a technique of performing full-thickness corneal transplantation where a diseased cornea is removed and replaced with a healthy and viable donor corneal button.[1] Eduard Konrad Zirm was the first to perform a solid organ corneal transplant in 1905. He successfully completed the first full-thickness corneal transplant. The surgery was performed on a patient with bilateral alkali burns. Vladimir Petrovich Filatov, a Russian ophthalmologist, is known as the father of keratoplasty as he was the one who suggested that cadaveric cornea can be used as a donor cornea for performing keratoplasty.[2]
History of Corneal Transplantation
In 1813, K. Himly made a significant contribution to the field of corneal transplantation by proposing the concept of replacing a cloudy or opaque cornea from one animal with the cornea of another animal. This pioneering work in animal corneal transplantation laid the foundation for further advancements in the field of corneal surgery and transplantation.[3] In 1824, F. Reisinger coined the term keratoplasty, and he was the first to suggest that the human cornea could be replaced by animal corneal tissue. In 1837, SLL Bigger first performed successful allograft transplantation in animals. In 1872, Henry Power first experimented with corneal grafting. The term lamellar keratoplasty was coined by Von Hippel in 1880, and he was the first to invent the circular trephine. In 1908, Plange first performed auto keratoplasty. From 1910 to 1950, VP Filatov did significant work in keratoplasty; he conducted the first systemic study in keratoplasty. He also suggested that cadaveric corneas can be used as donor cornea. From 1930-1950, Castroveijo designed numerous instruments for corneal microsurgery. In 1944, R Paton was the first to establish an eye bank in the USA. In 1950, Paufique and Charleux were the first to give the concept of lamellar keratoplasty and the concept of limbal and eccentric grafts. In 1954, Cott first performed the transplantation with the help of cryopreserved cornea. In 1960, Maumenee gave the concept of Graft rejection.[4]
In 1965, Troutman designed the surgical instrument and microscope in 1968. Maurice developed the first specular microscope. In 1974 came the concept of storage media, where B McCarey and H Kaufman developed the first corneal storage media. In 1985, air bubble-assisted DALK dissection was performed by Archila EA. In 1998, Melles performed the DALK and posterior lamellar keratoplasty. In 2001, Mark Terry first performed deep lamellar endothelial keratoplasty (DLEK). In 2006, Price and Gorovoy proposed the concept of Descemet's stripping endothelial keratoplasty (DSEK) and Descemet's stripping automated endothelial keratoplasty (DSAEK). In 2006, Melles first performed the Descemet membrane endothelial keratoplasty (DMEK).[5]
Anatomy and Physiology
The cornea is relatively immune privileged. The three factors which govern the success of keratoplasty are the absence of blood vessels, the absence of lymphatics, and anterior chamber-associated immune deviation (ACAID).[6]
Indications
Clinical Indications
- Pseudophakic bullous keratopathy (pseudophakic corneal edema)
- Aphakic bullous keratopathy (aphakic corneal edema)
- Corneal stromal dystrophies
- Granular
- Lattice
- Macular
- Central crystalline dystrophy of Schnyder
- Central cloudy dystrophy of Francois
- Corneal endothelial dystrophies
- Fuchs
- Congenital hereditary endothelial dystrophy
- Posterior polymorphous dystrophy
- Iridocorneal endothelial syndrome
- Chandler syndrome
- Corneal ectasia
- Anterior keratoconus
- Posterior keratoconus
- Keratoglobus
- Congenital opacities
- Peter anomaly
- Sclerocornea
- Aniridia
- Congenital glaucoma
- Viral keratitis
- Herpes simplex
- Adenovirus
- Herpes zoster
- Microbial keratitis
- Bacterial
- Fungal
- Chlamydial
- Infectious crystalline keratopathy
- Trachoma
- Parasitic
- Acanthamoeba
- Nutritional
- Chemical injuries
- Corneal degenerations
- Spheroidal degeneration
- Terrien marginal degeneration
- Band shaped keratopathy
- Traumatic corneal scar
- Ulcerative keratitis (non-infectious)
- Keratoconjunctivitis sicca
- Sjogren syndrome
- Exposure keratopathy
- Neurotrophic or neuroparalytic keratopathy
- Mooren ulcer
- Repeat graft
- Graft rejection
- Post-TPK
- Primary graft failure
- Regraft due to allograft rejection[7]
Penetrating Keratoplasty Types
- Tectonic: To restore the anatomical integrity of the globe
- Therapeutic/ reconstructive: To eliminate the infective load from the eye
- Cosmetic: Keratoplasty is done to remove the corneal opacity
- Optical: Keratoplasty is done to restore vision[8]
This section is focused on optical and penetrating keratoplasty. Therapeutic and tectonic keratoplasty has been discussed by the authors in another chapter previously,
Common Indications
- Aphakic bullous keratopathy
- Pseudophakic bullous keratopathy[9]
- Traumatic corneal scar
- Scar post-infective keratitis
- Corneal degenerations
- Stromal dystrophies
- Endothelial dystrophies
- Advanced keratoconus
- Congenital corneal opacities
Indication Based on Prognostic Outcomes
Category 1 - Excellent prognosis with more than 90% success rate
- Keratoconus
- Corneal dystrophy
- Fuchs dystrophy
Category 2 - Very good prognosis with 80 to 90% success rate
- Pseudophakic bullous keratopathy
- Aphakic bullous keratopathy
- Fuchs dystrophy
- Macular corneal dystrophy
- Interstitial keratitis
- Herpetic keratitis
- Iridocorneal endothelial syndrome[11]
Category 3 - Fair prognosis with 50 to 80% success rate
- Keratoglobus
- Pellucid marginal degeneration
- Active keratitis
- Corneal perforation
- Congenital hereditary endothelial dystrophy
- Corneal opacity in the pediatric age group
- Mild grade of dry eyes
- Mild grade of chemical injury[12]
Category 4 - Poor prognosis with less than 50% success rate
- Ocular pemphigoid
- Steven Johnson syndrome
- Congenital glaucoma
- Anterior chamber cleavage syndrome
- Multiple graft failures
- Neuroparalytic disease
- Neurotrophic keratitis[13]
Contraindications
- Severe dry eyes
- Steven johnson syndrome
- Toxic epidermal necrolysis
- Advanced ocular surface disease
- Anterior staphyloma
- Retinal detachment
- Blepharitis
- Meibomian gland disease
- Acute conjunctivitis
- Episcleritis
- Scleritis
- Corneal vascularization in more than two quadrants[14]
Equipment
- Drape
- Povidone Iodine
- Artery forceps
- Povidone iodine-soaked cotton balls
- Clean cotton balls
- Lid speculum
- Side port blade-15 degree
- MVR blade
- Conjunctival forceps
- Radial keratotomy marker
- Scleral ring
- Trephine
- Conjunctival scissor
- Vannas scissors
- Teflon block for graft trephination
- 10-0 and 9-0 nylon sutures
- Suture holding forceps
- Plain suture-tying forceps
- Capsulotomy needle: cystitome
- Capsulotomy forceps
- Sinskey hook
- Simcoe cannula
- Intraocular lens
- Automated anterior vitrectomy machine[15]
Personnel
- Ophthalmic Surgeon
- Mid-level ophthalmic assistant (MLOA)
- Operating room nurses
- Pharmacist
- Counseling nurses
- Eye bank coordinator
- Eye bank-trained technician
- Trained staff for enucleation
- Driver
- Sponsors
- Enucleation coordinator
Preparation
History
A thorough history is mandatory in each. The presenting complaint in most cases is defective vision and whitish opacity in each case. A prior history of ocular infection, history of ocular surgery, retinal pathology, duration of corneal opacity, amblyopia, and visual acuity before surgery or opacity. A history of ocular injury, infectious pathology, nutritional deficiency, collagen vascular disease, chronic ocular surface disease, history of herpetic keratitis, and systemic comorbidity must be documented.[16]
Ocular Examination
Visual Acuity
Every patient must undergo Snellen's uncorrected, best-corrected, and pinhole visual acuity evaluation. In cases with the perception of light, the projection of rays must be checked in all four quadrants. If the patient is unable to decipher, it can be evaluated with an indirect ophthalmoscope.
A stenopic slit can be used to evaluate visual acuity in cases with small central corneal opacity. In some cases, visual acuity can be taken after pupillary dilatation.
In children resistant to visual acuity evaluation, occlusion, preferential looking test, and Cardiff acuity can be used to check the visual acuity.[17]
Ocular Examination
Slit Lamp Biomicroscopy
Anterior Segment
Anterior segment examination is a must in every case to rule out local lid pathologies such as trichiasis, distichiasis, lacrimal gland pathology, blepharitis, meibomian gland disease, ectropion, entropion and signs of any infection such as conjunctivitis.[18]
The tarsal and bulbar conjunctiva, episcleral, and sclera should be evaluated for the presence of foreign bodies, inflammation, or signs of infection. The cornea should be assessed for size, shape, extent, and severity of corneal opacity, degree, and extent of corneal vascularization, and corneal sensations. In the case of a previous graft, the graft size, presence of any sutures, and graft characteristics must be documented. The anterior chamber depth, presence and absence of anterior synechiae, and clock hour of synechiae must be documented. Pupillary dilatation, presence of posterior synechiae, pupil characteristics such as occlusion pupillae, seclusio pupillae, or festooned pupil.
The lens status should be documented, whether phakic, aphakic, or pseudophakia. Phakic patients require cataract removal and IOL implantation, and the aphakic patient may require anterior vitrectomy, pupiloplasty and posterior chamber, iris claw, or SFIOL implantation.[19]
Posterior Segment
Posterior segment examination is mandatory in each case to look for the retinal status and rule out glaucoma. The disc, macula, retinal arcades, and periphery should be thoroughly evaluated. In patients with limited view, a B scan ultrasound should be done to rule out any vitritis, vitreous hemorrhage, glaucomatous cupping, and retinal or choroidal detachment.[10]
Intraocular Pressure Evaluation
The intraocular pressure should be evaluated in each case. In cases where non-contact tonometry gives an error, Mackay Marg, pneumotonometer, scleral tonometer, or Tono-Pen should be used. If these devices are not available, then digital tension should be assessed.[20]
Dry Eye Workup
Dry eye evaluation must be performed by documenting the tear film break-up time, staining pattern, and height by noticing the meniscus at the lower lid and assessing the Schirmer's value.[21]
Refraction
Objective and subjective refraction must be performed in each case.[22]
Gonioscopy
In needed cases with PAS formation suspected of synechial angle closure.[23]
Keratometry
In cases that undergo IOL implantation along with penetrating keratoplasty, it is essential to assess the keratometry to ascertain the IOL power. When keratometry cannot be calculated, the other eye IOL power is chosen, or normal standard keratometric values are taken for the calculation. Keratometric values also give an idea about irregular astigmatism.[24]
Pachymetry
Pachymetry gives an idea of the corneal thickness needed for documentation.[25]
Anterior segment Optical Coherence Tomography (ASOCT)
ASOCT gives an idea about the opacity depth, that is, whether it is anterior stromal, posterior stromal, or extending. ASOCT helps plan for either full thickness graft or a lamellar graft.[26]
Ultrasound biomicroscopy (UBM)
In a few cases, ultrasound biomicroscopy may be required to look for the angle and ciliary body characteristics.[27]
Tissue Storage
The death to enucleation time is 6 hours. The storage of corneal tissue is extremely important as the outcome of penetrating keratoplasty depends on the quality of corneal tissue and the endothelial cell count. For OPK, the tissue should be ideally used within 48 hours, and the endothelial cell count should be at least more than 2000 cells. The storage media available are a moist chamber for 48 hours, M-K media for up to four days, cornisol media for 7 to 10 days, organ culture for 35 days, and cryopreservation for up to one year.[28]
Preoperative Tissue Evaluation
A preoperative tissue evaluation form is done before keratoplasty. The corneal tissue is assessed in the format of corneal epithelium, bowman, stroma, Descemet, and endothelium. The epithelium is assessed for defects, haze, slough, and exposure. The stroma is assessed for any opacity, arcus senilis, and compactness. The Descemet is assessed for any folds, edema, detachment, guttae, or defect. The endothelium should be assessed for clarity and compactness.[15]
Finally, a specular count should be done, and tissue should be labeled whether fit for the research, wet lab, TPK, lamellar, or OPK.
The various contraindications for donor button selection are
- Death due to an unknown cause
- Rabies
- CNS pathologies like Creutzfeldt-Jacob disease, Subacute sclerosing panencephalitis (SSPE), and progressive leukoencephalopathy.
- AIDS
- Septicemia
- Systemic infections like syphilis, hepatitis B and C, CMV, EBV
- Intraocular tumors
- Leukemia and lymphoma[29]
Preoperative Preparation of the Eye
If there is a presence of blepharitis or MGD, the adnexal infection should be treated first. If corneal neovascularization is present, it should be treated with preoperative topical steroids, electrocautery, adrenaline-soaked sponges, or argon laser photocoagulation. The ocular pressure should be controlled preoperatively by either intravenous mannitol or a Honan's balloon. It helps to decrease the vitreous pressure during the open sky procedure, which reduces the vitreous loss and risk of expulsive choroidal hemorrhage. Pilocarpine is injected in cases of phakic OPK, and tropicamide in patients with cataracts to dilate the pupil.[30]
Technique or Treatment
Eyeball Painting and Draping
The eye is painted with a 5% povidone-iodine solution and dried with cotton using artery forceps. Then drape is applied, and the eyeball is exposed using a lid speculum.[31]
Paracentesis
Initially, a small paracentesis is made, and pilocarpine is injected if the lens is clear and needs to be preserved. If cataract removal is also attempted, tropicamide is injected, followed by 0.06% trypan blue and viscoelastic substance.[32]
Host Trephination
First of all central cornea is marked with blue ink of a marker. A caliper or a sinkey hook having blue ink can be used to mark the center of the cornea. The host marking is usually kept at 7 to 7.5 mm, and a 7 to 7.5 mm trephine marks the cornea. A radial keratotomy dipped in ink can also make markings over the host cornea.[33]
The cornea is dried before marking the host cornea. The trephine is gently rotated between the thumb and the forefinger up to 80% depth of the cornea. Care should be taken to avoid 100% depth of the cornea to prevent injury to the iris and lens diaphragm. An MVR blade is used to do a guarded entry at the trephine site, and then a corneoscleral or vannas scissor is used to enter the anterior chamber. One should remember that the small the graft, the more astigmatism and the less the chance of rejection. The larger the graft, the less astigmatism and rejection is more possible.[34]
Donor Corneal Button Preparation
The graft is taken out from the storage media in a petri dish. The donor button is held with conjunctival forceps, and the scleral rim is dried with a cotton sponge preventing damage to the endothelium. Moreover, the iris remnants can be removed using a dried cotton bud or cotton piece.[35]
The donor button is kept on the Teflon block and punched with pressure downwards. Care should be taken so that the Teflon block doesn't slip. The graft size is usually kept 0.5 mm larger than the host except in cases with keratoconus, where the graft size is kept the or 0.25 mm larger to compensate for myopia.
The majority of surgeons use hand-held trephines. The various punches available are Cottingham's punch, Barron vacuum corneal donor punch, IOWA PK press corneal punch, and Rothman Gilbard corneal punch. The excimer laser can also be used to trephine the donor corneal button.[36]
If the host dissection is larger than 9 mm and smaller than 7 mm, the graft should be kept 1 mm larger than the host. If the host dissection is between 7 and 9 mm, the graft should be kept 0.5 mm larger than the host. In the case of aphakia, it is recommended to keep the graft size more than 0.5 mm, and in case it is phakic, the diameter should be kept at 0.25 mm larger.
Anterior Chamber Entry
The controlled anterior chamber should be done under viscoelastic cover, and peripheral anterior synechiae, if any, should be released.[37]
Peripheral Iridectomy
A peripheral iridectomy (PI) should be done with the help of vannas scissors and forceps. A PI can bleed hence care should be taken to avoid the formation of blood clots. If there is a bleed, adrenaline can be injected to stop the bleeding, and the anterior chamber should be washed with saline to remove the bleeding or any blood clot.[38]
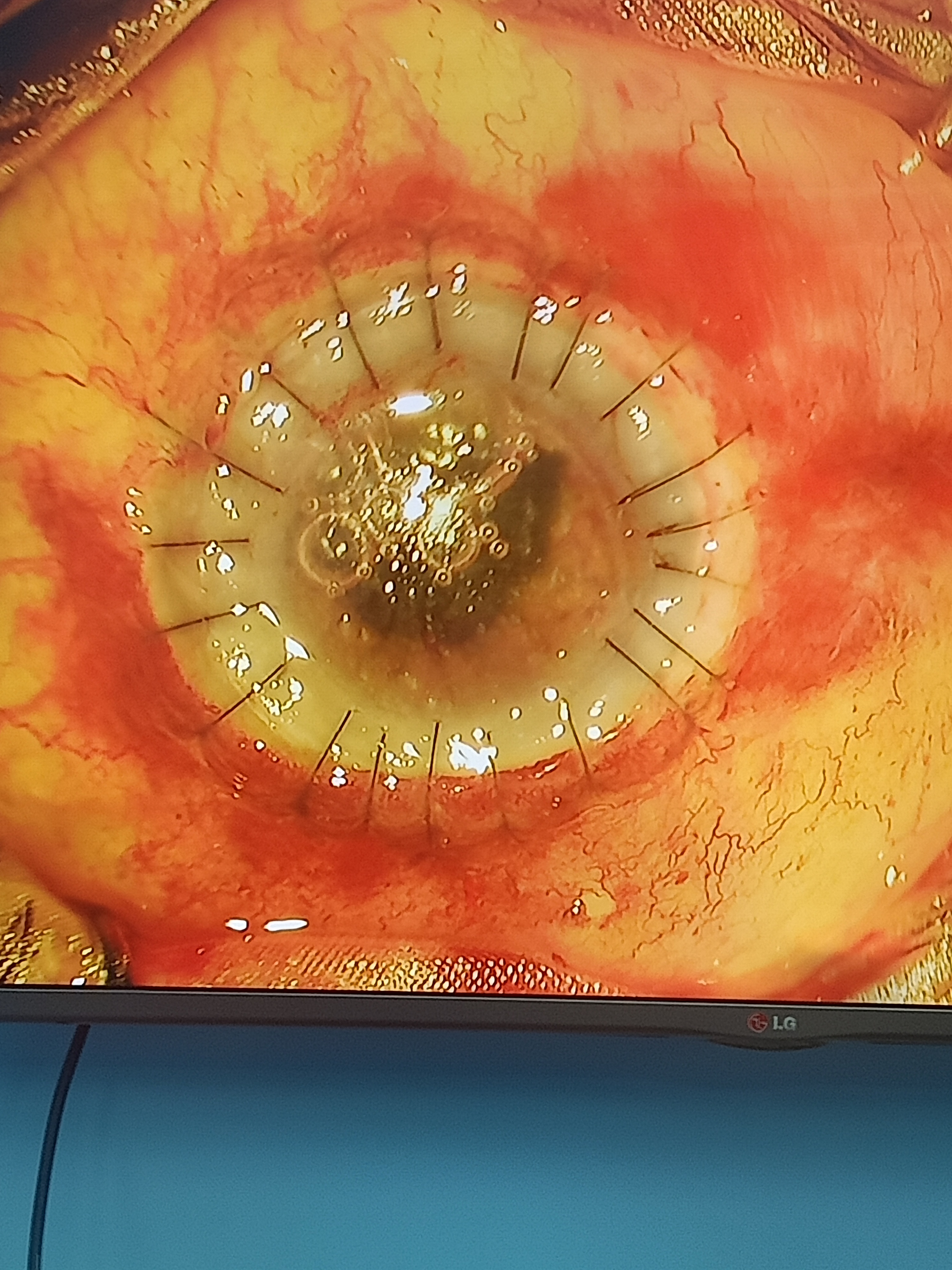
Suturing of the Donor Tissue to the Host Rim
The donor tissue should be sutured to the host rim with 10-0 nylon sutures. Initially, 4 cardinal sutures are placed at 12, 6, 9, and 3 o'clock positions. The 4 cardinal sutures should form a square.
There are 4 suturing techniques:
- Single interrupted suturing technique
- Combined continuous and interrupted suturing technique
- Single continuous suturing technique
- Double continuous suturing technique[39]
The single continuous suturing technique has 3 different subtypes
- Torque - this rotates the graft counter-clockwise 0.7 ± 0.1 mm at the wound or 11 degree
- Antitorque - Rotate the graft clockwise 0.7 ± 0.1 mm at the wound or 11 degree
- No torque - By this technique, an isosceles triangle is formed, which produces no rotational effect[40]
Advantages of Interrupted Sutures
- They are recommended in children and infants, highly vascularized corneas, and patients with therapeutic keratoplasty.
- This gives the advantage of selective suture removal.
- The needle should pass just above the Descemet membrane. The suture length should be 2 mm on the host site and 1 mm on the donor site.
- Full-thickness sutures should be avoided as they damage the endothelium, and aqueous can leak through the suture tract postoperatively.
- The suture knots should ideally be buried on the host site because it would invite vascularization if buried on the recipient site.[39]
Postoperative Management
Corticosteroids
In OPK, postoperatively, the patient should be started hourly on topical steroids (1% prednisolone or 0.1% dexamethasone) for the first 2 days. Then the steroid regimen should be tapered 6 times for 15 days, and then 4/3/2/1 times 3 months each. For the first 3 days, intravenous steroids such as methylprednisolone should be injected at 1 g twice daily. This should be followed by oral steroids (prednisolone) in a tapering regimen of 40/30/20/10 mg for 3 to 5 days each. The steroid regimen can be altered based on the patient's clinical response.[41]
Antibiotics
Antibiotics should be given 4 to 6 times daily for the first 2 weeks to prevent secondary bacterial infection. In cases with graft infection, antibiotics may be required for a prolonged period.[42]
Antiglaucoma Medications
In these cases, adjuvant drugs like timolol, betaxolol, or other antiglaucoma are required to counteract secondary glaucoma. Antiglaucoma medications are also helpful in pre-existing glaucoma and OPK with combined procedures such as vitrectomies, cataract surgery, synechiolysis, hyaluronidase use, synechiaolysis, and anterior segment reconstruction. Topical prostaglandin use is controversial. Dorzolamide affects the corneal endothelium and may result in prolonged graft edema and should be avoided post-keratoplasty.[43]
Cycloplegics
Cycloplegic drugs like topical homatropine, cyclopentolate, and atropine should be added for the initial period to relieve pain and ciliary spasm. Cycloplegics should be used cautiously as there is a risk of Urretz-Zavalia syndrome.[10]
Lubricants
Topical preservative-free lubricants should be used to prevent suture-induced irritation.
Complications
Preoperative
- Inadequate analgesia
- Inadequate anesthesia
- Positive vitreous thrust
- Lens expulsion
- Vitreous prolapse
- Block-induced corneal perforation
Intraoperative
- Scleral perforation- during application of superior rectus suture
- Trephination- Eccentric trephination, reversed host, and donor trephination.
- Oval trephination
- Irregular trephination
- Iridodialysis
- Retained Descemet's membrane- especially in cases with a thick cornea, such as congenital hereditary endothelial dystrophy and interstitial keratitis. Failure to grasp this iris is a sign of retained Descemet membrane.
- Corneal button damage
- Inversion of the graft
- Torrential bleeding
- Iris lens diaphragm injury
- Lens expulsion
- Vitreous prolapse
- Retained cortical matter
- Posterior capsular rent
- Tight sutures
- Superficial sutures
- Irregular suture bites
- Full-thickness suture bites
- Iris's incarceration in suture bites
- Expulsive choroidal hemorrhage
Postoperative
Early
- Wound leak
- Wound dehiscence
- Shallow anterior chamber
- Iris's incarceration in wound
- Suture related complications
- Loose suture
- Suture infiltrate
- Suture induced vascularization
- Secondary glaucoma
- Descemet membrane detachment
- Vitreous in the anterior chamber
- IOL dislocation
- IOL subluxation
- Peripheral anterior synechiae
- Urretz-Zavalia syndrome
- Posterior synechiae
- Endophthalmitis
- Panophthalmitis
Late
- Graft rejection
- Graft failure
- Graft infiltrate
- Loose sutures
- Suture infiltrate
- Hypopyon
- Secondary glaucoma
- Endophthalmitis
- Descemet membrane detachment
- Filamentary keratitis
- Microbial keratitis
- Hyphema
- HSV keratitis
- Epithelial defect
- Astigmatism
- Cataract
- Corneal neovascularization
- Retrocorneal membrane
- Retinal detachment
- Choroidal detachment
- Macular edema
- Endophthalmitis
- Panophthalmitis
- Infectious crystalline keratopathy[44]
Clinical Significance
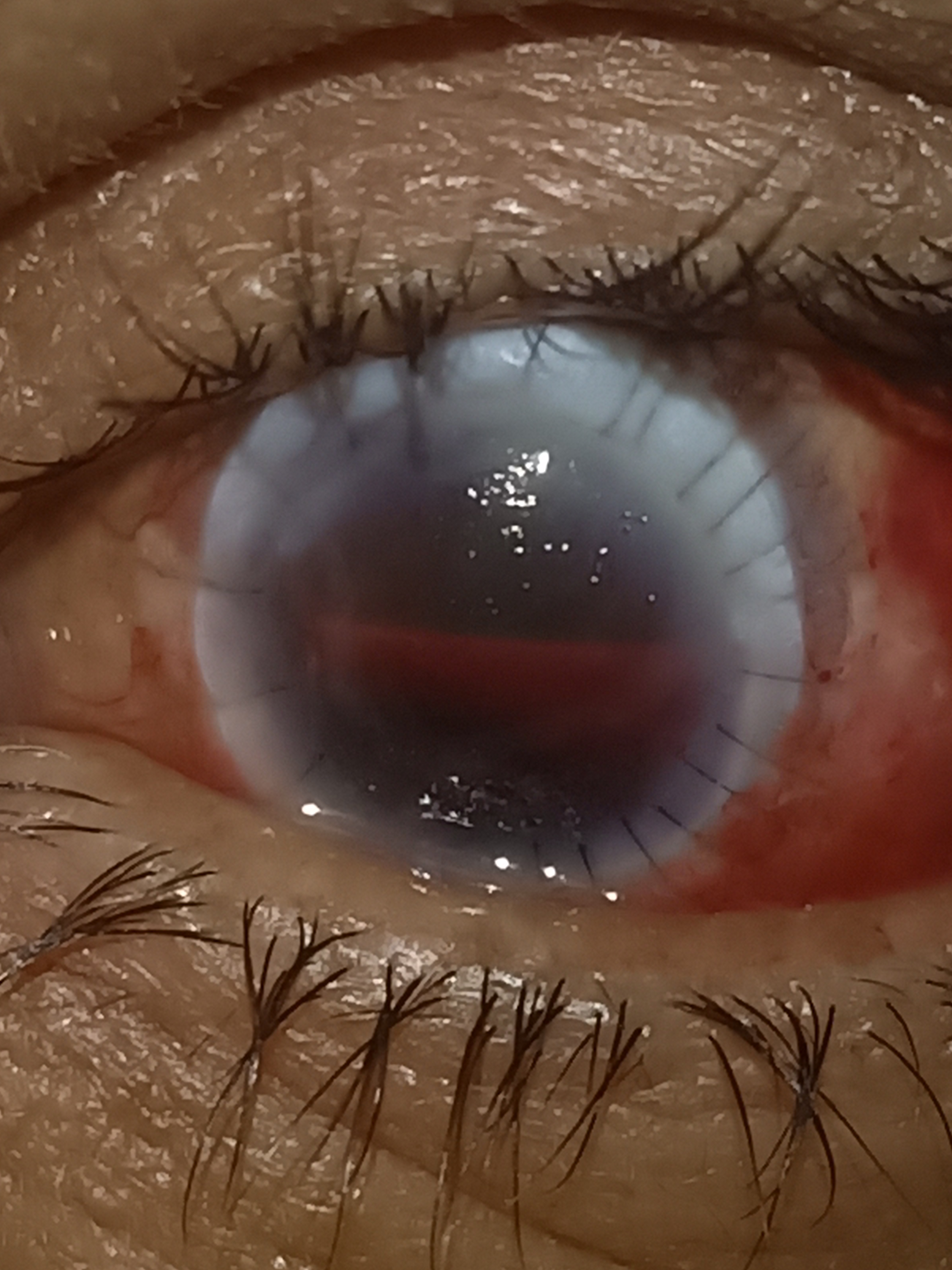
Optical penetrating keratoplasty plays a considerable role in visually rehabilitating blind and needy patients. The visual recovery takes place slowly over 3 or 4 months. OPK remains the gold standard for most corneal pathologies, and patients should be explained the prognosis before embarking on the procedure.[45]
R.P. Centre Grading of Graft Clarity
- 0-Totally Opaque Graft
- 1-Very hazy cornea graft, iris, lens, and anterior chamber details barely visible
- 2-The graft is hazy, the iris, lens, and anterior chamber details are visible, but details are not clear
- 3-The graft is clear, but some details of the iris and lens obscured
- 4-The graft is clear, and all details of the anterior chamber and lens are visible
Difference between Immune Suture Infiltrate and Infectious Suture Infiltrate
|
S. No
|
Characteristics
|
Immune Suture Infiltrate
|
Infectious Suture Infiltrate
|
|
1
|
Location
|
Host side only
|
Graft or host side both
|
|
2
|
Number
|
Multiple and small
|
Solitary
|
|
3
|
Epithelial defect
|
May or may not be present
|
Epithelial defect common
|
Enhancing Healthcare Team Outcomes
Any patient with corneal opacity should be evaluated in detail for the presence or absence of visual potential. The eye should be assessed for the prognosis if there is vision potential. The risk factor for poor prognosis should be labeled.[46] The patient should be evaluated for compliance and follow-up. In case of lack of compliance decision for keratoplasty should be reconsidered. The patient should be clearly explained the nature and outcome of the keratoplasty. There are also surgeon-related factors and tissue quality which govern the outcome. The surgeon should perform a meticulous keratoplasty with minimal handling of the endothelium. All sutures should be equally spaced with equal tightness. The nursing staff, operating room staff, counselor, and pharmacist have a key role in governing the safety and outcome of these cases.[18] These various professionals can coordinate their activities as an interprofessional team to achieve optimal patient outcomes. [Level 5]
Nursing, Allied Health, and Interprofessional Team Interventions
The nursing staff, allied health staff, and the interprofessional team play a key role in patient recruitment, helping the operating surgeon in the operating room, assisting the surgeon during suture removal, and patient follow-up.[47]
Nursing, Allied Health, and Interprofessional Team Monitoring
The nursing staff, allied health staff, and the interprofessional team also play a crucial role in patient monitoring intraoperatively and postoperatively. The staff plays a key role in patient counseling, follow-up, and regular monitoring of systemic parameters.[48]