Introduction
Our skin serves many functions - including protective, sensory, temperature regulation, and fluid homeostasis. The outermost layer, the epidermis, is primarily involved in protective functions both as a physical and an immunologic barrier. These functions can all be correlated histologically, and derangements of these functions have clinical ramifications. This article will cover the structure and function of the normal epidermis. It will also briefly describe tissue preparation, immunohistochemistry, light microscopy, and electron microscopy. Lastly, it will describe some pathologies and select clinical and histologic correlations.
Structure
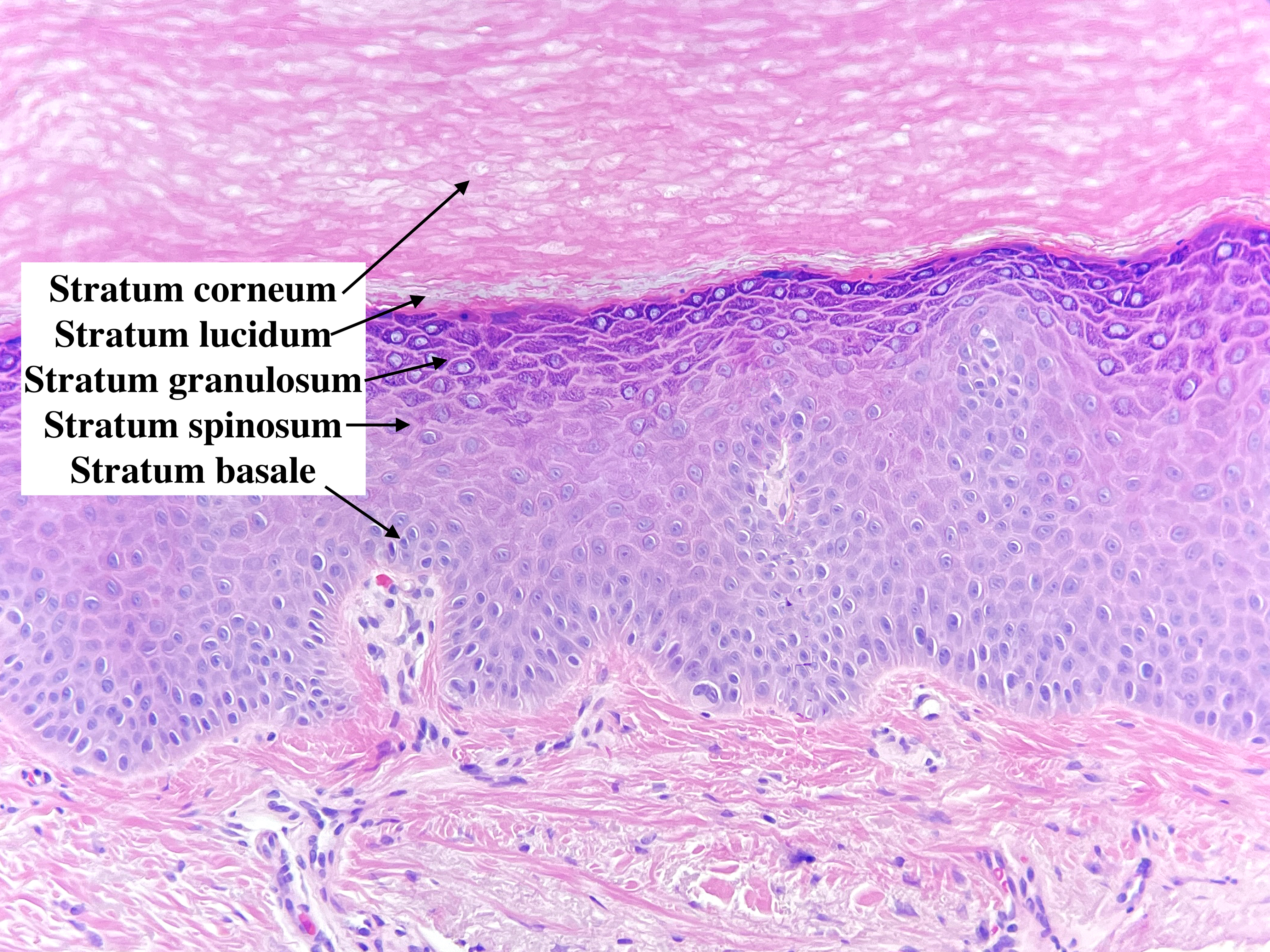
The normal epidermis can be broken down into five distinct layers: the stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. The stratum basale contains the stem cells for keratinocytes that constantly regenerate and push keratinocytes further outwards. As the keratinocytes mature, they travel from stratum to stratum with alterations in key characteristics. The cells are held together with intercellular connections called desmosomes. Under certain pathologic conditions like spongiosis, the desmosomes are visible and appear as spines between cells - which is where the stratum spinosum derived its name.
As cells rise through the stratum spinosum, keratinocytes begin to flatten out, and keratohyalin granules become readily apparent, giving the next layer the stratum granulosum. Other granules are present, including the lamellar granules, which contain glucosylceramides and an assortment of lipids and proteins that are eventually used to bond cells together.[1] The stratum lucidum is found more prominently on acral skin or any skin that is irritated and chronically scratched. The outermost layer of the skin is the stratum corneum, where the cells eventually flake off.
In normal skin, our epidermis contains four indigenous cell populations: keratinocytes, melanocytes, Langerhans cells, and Merkel cells.
Keratinocytes comprise the bulk of the epidermis and originate from stem cells in the basal layer. After formation, they mature as they rise through the epidermal layers. Their endpoint is the outermost layer, the stratum corneum. By the time they reach this layer, the keratinocytes will have shed their nuclei and flattened out to create the cemented outer layer of our epidermis. The differentiation of a keratinocyte from the basal layer through the flaking off of the stratum corneum takes approximately four weeks.[2][3] Keratinocytes have altered rates of maturation and replication in certain pathologic conditions. Keratinocytes stain with cytokeratin and p63 stains, among others.
Melanocytes, under normal conditions, are found intermittently studded in the basal layer. Melanocytes synthesize and store melanin in organelles known as melanosomes. Melanocytes transfer melanosomes via dendrites, primarily to keratinocytes.[4] The melanin content in the skin is responsible for variations in pigmentation. Altered distributions of melanocytes or destruction of melanocytes occur in various pathological conditions leading to hypopigmented areas of skin. Melanocytes are found in the basal layer at a ratio of roughly one melanocyte to 10 keratinocytes when evaluating a histological slide in two dimensions or a ratio of 1 melanocyte to roughly 40 keratinocytes in 3D space. Melanocytes have a variety of stains, but some of the most common ones are S100, Mart-1, MITF, and SOX10.
Langerhans cells, derived from monocytes, are the principal antigen-presenting cells found in the epidermis and the most prevalent immune cell type alongside CD8+ T cells.[5] Their function is primarily immune surveillance. The ratio of Langerhans cells to keratinocytes in normal skin is roughly 1 Langerhans cell to 53 keratinocytes. Each Langerhans cell has dendrites that weave through the keratinocytes; these dendrites absorb and metabolize antigenic proteins into peptides. These peptides can then be carried to the regional lymph node with Langerhans cells. Langerhans cells stain with S100, CD1a, and Langerin.
Merkel cells are neuroendocrine cells of epidermal origin that are involved in sensation.[6][7] Functionally, they act as slow-adapting mechanoreceptors. They exist predominantly in the basal layer of the epidermis and can be identified with a cytokeratin 20 stain.
Function
The skin offers a variety of functions, and an exhaustive list would deserve an article of its own. The functions of the epidermis include but are not limited to physical barrier protection from the environment, regulating circulation, immunologic protection from infectious etiologies, and protection of DNA.
The scaffolding of dead keratinocytes in the stratum corneum holds in place a hydrophobic lipid matrix from the granules in the keratinocytes. This serves both as a physical protective barrier that is constantly regenerating as well as a means of regulating electrolyte homeostasis and preventing transepidermal water loss.[8]
As foreign microbes attempt to invade, they will be first blocked by the barrier of the stratum corneum. As bacteria and fungi are able to penetrate further, they will be picked up by Langerhans cells for antigen presentation.[9]
The protection of DNA is a byproduct of skin pigmentation. Pigmentation of the skin is derived from melanocytic activity.[10] Increased melanin production and, by proxy, increased pigmentation have protective effects against ultraviolet-radiation-induced DNA damage.[11] However, increased pigmentation does have the tradeoff of less cutaneous vitamin D production.[12]
Tissue Preparation
Tissue preparation varies based on the intended use, but there are specific steps that are universal. All samples need to be clearly labeled and marked with unique identification numbers that tie the piece back to the particular patient. Gross examination and dissection also change based on the reason for the biopsy. For example, in biopsies taken to evaluate alopecia, different methods are used to obtain horizontal and/or vertical sections. Horizontal sections are particularly useful for evaluating the total number of hair follicles and assessing the proportion of hairs of different sizes and growth cycle phases. Vertical sections are better for evaluating the epidermal changes and the level of the pathologic changes.
Once the tissue has been dissected, fixation is the method whereby tissue is preserved, hardened, and infectious agents are devitalized. This process can alter the protein structure and shrink the tissue. Artifacts of tissue preparation are sometimes used as a diagnostic clue; for example, basal cell carcinomas tend to show retraction clefting around the palisading lesions under light microscopy.[13]
Histochemistry and Cytochemistry
Immunohistochemistry (IHC) is a process that involves using selective antibodies to target and stain certain antigens known to be specific to a particular condition. This creates a color signal that can be visualized on light microscopy. IHC can be used both for diagnostic and prognostic purposes. Immunohistochemical stains are routinely used to help diagnose melanocytic, hematolymphoid, histiocytic, vascular, and cutaneous adnexal neoplasms.[14][15]
However, their use has limitations because of the variability in sensitivity and specificity. Therefore, in most cases, multiple stains must be used to narrow the diagnostic possibilities. For example, in the diagnosis of melanocytic tumors, some stains such as S100, SOX10, and Mart-1 stain are highly sensitive and are often used not only to confirm the lineage of the neoplasm but also to highlight its overall architecture.
Another stain, PRAME is mostly positive in melanoma; however, it is not completely sensitive and can be falsely positive in some nevi. Regarding prognosis, the proliferative marker Ki-67 stain has been shown to be associated with prognosis, as it is a marker associated with the vertical growth of melanoma.[16]
Microscopy, Light
Light microscopy is the primary means for the magnified examination of tissue blocks. Most microscopes used in dermatopathology are compound microscopes. Compound microscopes offer two locations of magnification, the eyepiece, which traditionally has 10x magnification, and the objective lens, which is situated directly above the slide. Different microscopes can have varying magnification settings, but a standard microscope generally offers 10x, 40x, and 100x with objective lens magnification. The 100x magnification usually requires a medium between the lens and the slide, such as oil immersion. Light microscopy also generally shows cells as colorless, necessitating the need for various stains. Most tissue is initially stained with hematoxylin and eosin (H&E).
Microscopy, Electron
The electron microscope was developed in the 1930s and had the comparative advantage of examining tissue down to 0.2 nm as opposed to 200 nm for light microscopy. Electron microscopy in diagnosing dermatologic diseases has been largely overshadowed by the advent of immunohistochemical staining.[17] Uses persist primarily in research as a means of examining tissue down to the scale of the organelles.[1]
Pathophysiology
Many inflammatory pathologic conditions are associated with disruptions in the local skin microbiota leading to pathology or changes in the skin itself, leading to altered relations with the local microbiota. Teenage acne vulgaris involves clogging pores secondary to sebaceous hyperplasia and lipid release into the follicular lumen, which is exacerbated by Cutibacterium acnes activation of keratinocytes.[18]
Other skin conditions that are genetic or innate in nature represent aberrations in the normal structure and function of the skin. For example, psoriasis is a chronic papulosquamous genetic skin disorder characterized by hyperproliferation in the epidermis stimulated by immune dysfunction. Certain T-cell immune cytokines, most notably IL-17 and IL-23, are upregulated. This leads to keratinocyte proliferation, neutrophil chemotaxis, and angiogenesis.[19]
Another prominent genetic condition that affects the epidermis is atopic dermatitis. This condition is driven by a mutation in Filaggrin, an element of the epidermis. This deficiency allows for increased colonization and penetration by the skin microbiome, which promotes inflammation and drives the characteristic pruritus.[20]
Clinical Significance
The microscopic examination of diseased skin entities reveals specific findings correlated to the clinical results. Dermatologic diseases have been broken into various histopathologic reaction patterns differentiated by changes throughout the skin, while various organizing schemes exist.[21] A select few patterns more characterized by epidermal changes will be briefly addressed here: spongiotic dermatitis, vesiculobullous dermatoses, psoriasiform disorders, and interface dermatitis.
The predominant histologic finding of spongiotic dermatitis is spongiosis, seen as intraepidermal edema. This widening of the spaces between the keratinocytes reveals the intercellular desmosomes. Some of the classic spongiotic disorders include atopic dermatitis and allergic contact dermatitis.[22]
The predominant histologic finding of vesiculobullous dermatoses is acantholysis, or loss of connection between cells at various levels of the epidermis, which correlates to the blisters seen clinically. This is more severe than the simple widening of spaces in spongiosis and represents the actual dehiscence of cells through autoimmune or infectious degeneration of desmosomes and hemidesmosomes.[23] Some classic vesiculobullous disorders include pemphigus vulgaris, Hailey-Hailey disease, and eczema herpeticum.[24]
The predominant histologic finding of the psoriasiform disorders is acanthosis, or epidermal hyperplasia, with concurrent elongation of the rete ridges, which correlates to the thickened plaques seen clinically. The quintessential example of a psoriasiform disorder is psoriasis vulgaris, but other examples include pityriasis rubra pilaris and pityriasis rosea.[25]
The previously mentioned keratinocyte proliferation, neutrophil chemotaxis, and angiogenesis correlate clinically with the thick plaques, microabscesses of Munro and pustules of Kogoj, and superficial vessels that are visible when the scale is peeled off. This pinpoint bleeding is named the Auspitz sign.
The predominant histologic finding of the interface disorders is the bandlike infiltrate of leukocytes abutting the epidermis that represents inflammation and is associated with concomitant dermo-epidermal junction changes such as basement membrane thickening, vacuolar degeneration of the basal cells, or apoptosis of keratinocytes. Some prominent examples include lichen planus, erythema multiforme, or dermatomyositis.[26]
All of these patterns can have degrees of overlap with one another and variation within the reaction pattern, especially depending on chronicity. An early, acute spongiotic dermatitis will appear vastly distinct from a chronic condition. Concurrent treatment of conditions will also alter the appearance histologically.