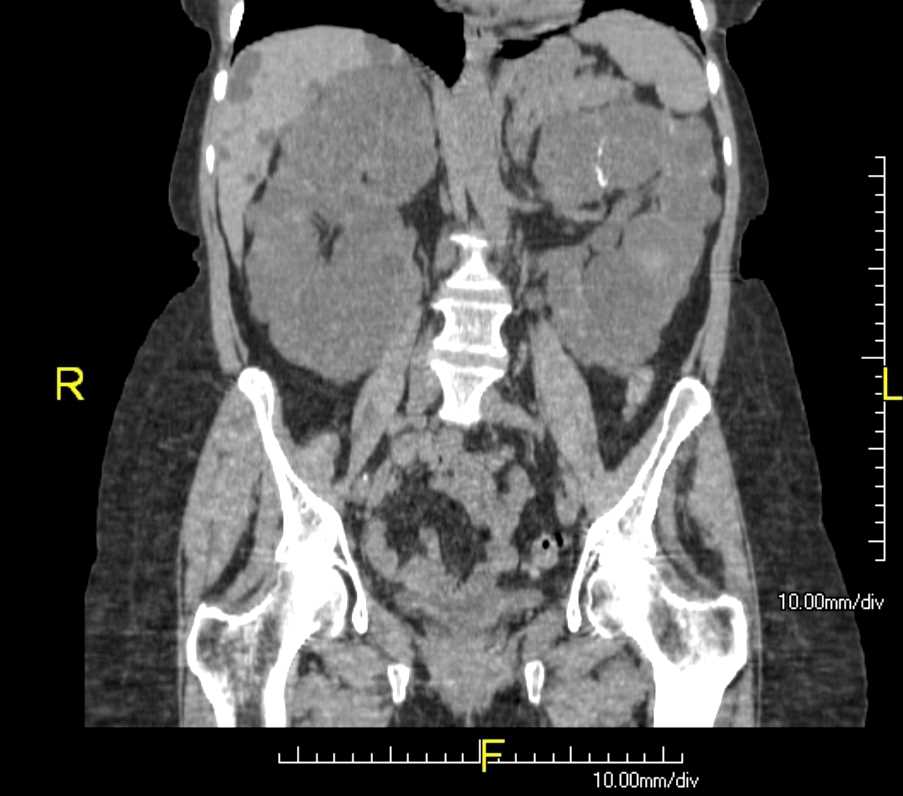
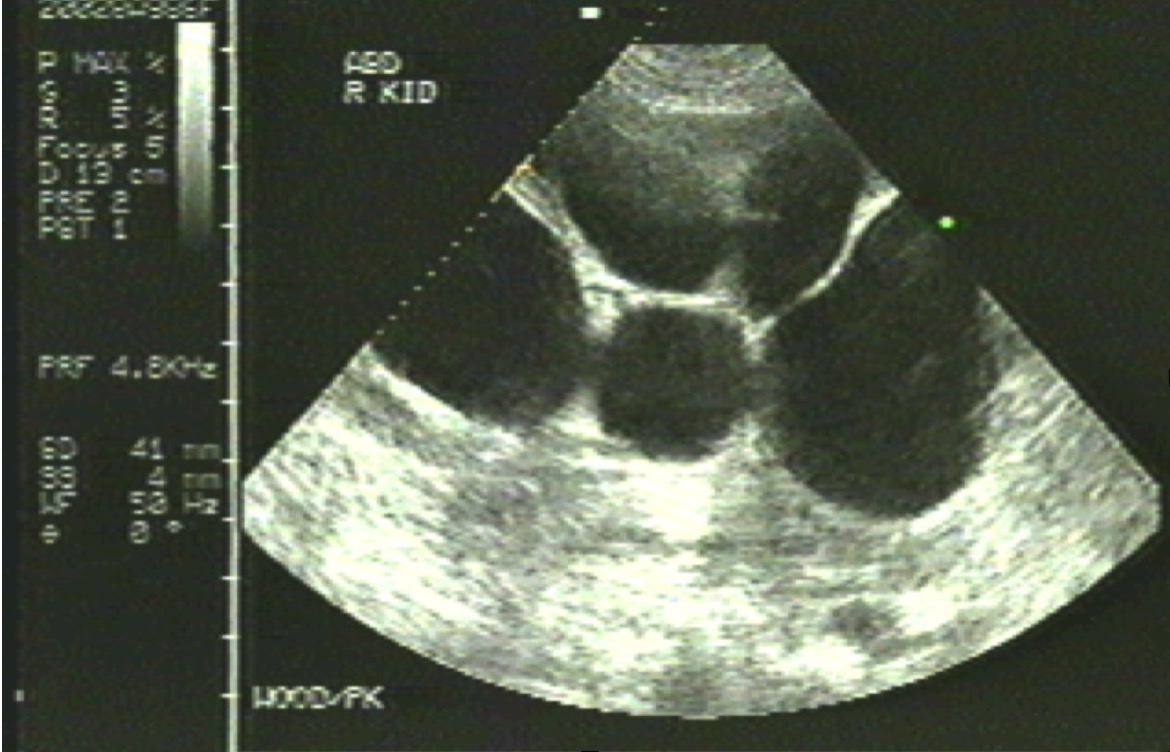
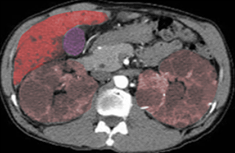
[1]
Pandita S, Khullar D, Saxena R, Verma IC. Autosomal Dominant Polycystic Kidney Disease: Presence of Hypomorphic Alleles in PKD1 Gene. Indian journal of nephrology. 2018 Nov-Dec:28(6):482-484. doi: 10.4103/ijn.IJN_236_17. Epub
[PubMed PMID: 30647506]
[2]
Cho Y, Sautenet B, Gutman T, Rangan G, Craig JC, Ong AC, Chapman A, Ahn C, Coolican H, Kao JT, Gansevoort R, Perrone RD, Harris T, Torres V, Pei Y, Kerr PG, Ryan J, Johnson DW, Viecelli AK, Geneste C, Kim H, Kim Y, Oh YK, Teixeira-Pinto A, Logeman C, Howell M, Ju A, Manera KE, Tong A. Identifying patient-important outcomes in polycystic kidney disease: An international nominal group technique study. Nephrology (Carlton, Vic.). 2019 Dec:24(12):1214-1224. doi: 10.1111/nep.13566. Epub 2019 May 2
[PubMed PMID: 30663163]
[3]
Zhang M, Srichai MB, Zhao M, Chen J, Davis LS, Wu G, Breyer MD, Hao CM. Nonselective Cyclooxygenase Inhibition Retards Cyst Progression in a Murine Model of Autosomal Dominant Polycystic Kidney Disease. International journal of medical sciences. 2019:16(1):180-188. doi: 10.7150/ijms.27719. Epub 2019 Jan 1
[PubMed PMID: 30662341]
[4]
AlNuaimi D, AlKetbi R, AlFalahi A, AlBastaki U, Pierre-Jerome C. Ruptured Berry Aneurysm as the initial presentation of Polycystic Kidney Disease: A case report and review of literature. Journal of radiology case reports. 2018 Sep:12(9):1-8. doi: 10.3941/jrcr.v12i9.3448. Epub 2018 Sep 30
[PubMed PMID: 30651918]
Level 3 (low-level) evidence
[5]
Steele C, You Z, Gitomer BY, Brosnahan GM, Abebe KZ, Braun WE, Chapman AB, Harris PC, Perrone RD, Steinman TI, Torres VE, Yu ASL, Chonchol M, Nowak KL. PKD1 Compared With PK D2 Genotype and Cardiac Hospitalizations in the Halt Progression of Polycystic Kidney Disease Studies. Kidney international reports. 2022 Jan:7(1):117-120. doi: 10.1016/j.ekir.2021.09.013. Epub 2021 Oct 7
[PubMed PMID: 35005320]
[6]
Harris PC, Torres VE. Polycystic kidney disease. Annual review of medicine. 2009:60():321-37. doi: 10.1146/annurev.med.60.101707.125712. Epub
[PubMed PMID: 18947299]
[7]
Liebau MC, Mekahli D, Perrone R, Soyfer B, Fedeles S. Polycystic Kidney Disease Drug Development: A Conference Report. Kidney medicine. 2023 Mar:5(3):100596. doi: 10.1016/j.xkme.2022.100596. Epub 2022 Dec 27
[PubMed PMID: 36698747]
[8]
Dell KM. The role of cilia in the pathogenesis of cystic kidney disease. Current opinion in pediatrics. 2015 Apr:27(2):212-8. doi: 10.1097/MOP.0000000000000187. Epub
[PubMed PMID: 25575298]
Level 3 (low-level) evidence
[9]
Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. Journal of the American Society of Nephrology : JASN. 2009 Jan:20(1):23-35. doi: 10.1681/ASN.2008050456. Epub 2008 Dec 31
[PubMed PMID: 19118152]
[10]
Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nature reviews. Molecular cell biology. 2007 Nov:8(11):880-93
[PubMed PMID: 17955020]
[11]
Malekshahabi T, Khoshdel Rad N, Serra AL, Moghadasali R. Autosomal dominant polycystic kidney disease: Disrupted pathways and potential therapeutic interventions. Journal of cellular physiology. 2019 Aug:234(8):12451-12470. doi: 10.1002/jcp.28094. Epub 2019 Jan 15
[PubMed PMID: 30644092]
[12]
Lee JJ, Cheng SJ, Huang CY, Chen CY, Feng L, Hwang DY, Kamp TJ, Chen HC, Hsieh PCH. Primary cardiac manifestation of autosomal dominant polycystic kidney disease revealed by patient induced pluripotent stem cell-derived cardiomyocytes. EBioMedicine. 2019 Feb:40():675-684. doi: 10.1016/j.ebiom.2019.01.011. Epub 2019 Jan 11
[PubMed PMID: 30639418]
[13]
Lanke S, Shoaf SE. Population Pharmacokinetic Analyses and Model Validation of Tolvaptan in Subjects With Autosomal Dominant Polycystic Kidney Disease. Journal of clinical pharmacology. 2019 May:59(5):763-770. doi: 10.1002/jcph.1370. Epub 2019 Jan 7
[PubMed PMID: 30618157]
Level 1 (high-level) evidence
[14]
Li X, Wüthrich RP, Kistler AD, Rodriguez D, Kapoor S, Mei C. Blood Pressure Control for Polycystic Kidney Disease. Polycystic Kidney Disease. 2015 Nov:():
[PubMed PMID: 27512778]
[15]
Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. The New England journal of medicine. 1990 Oct 18:323(16):1091-6
[PubMed PMID: 2215576]
[16]
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet (London, England). 2007 Apr 14:369(9569):1287-1301. doi: 10.1016/S0140-6736(07)60601-1. Epub
[PubMed PMID: 17434405]
[17]
Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D. Unified criteria for ultrasonographic diagnosis of ADPKD. Journal of the American Society of Nephrology : JASN. 2009 Jan:20(1):205-12. doi: 10.1681/ASN.2008050507. Epub 2008 Oct 22
[PubMed PMID: 18945943]
[18]
Barash I, Ponda MP, Goldfarb DS, Skolnik EY. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2010 Apr:5(4):693-7. doi: 10.2215/CJN.04180609. Epub 2010 Feb 18
[PubMed PMID: 20167686]
Level 3 (low-level) evidence
[19]
Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2009 Jun:4(6):1140-50. doi: 10.2215/CJN.00790209. Epub 2009 May 14
[PubMed PMID: 19443627]
Level 3 (low-level) evidence
[20]
Torres VE. Salt, water, and vasopressin in polycystic kidney disease. Kidney international. 2020 Oct:98(4):831-834. doi: 10.1016/j.kint.2020.06.001. Epub
[PubMed PMID: 32998813]
[21]
Arogundade FA, Akinbodewa AA, Sanusi AA, Okunola O, Hassan MO, Akinsola A. Clinical presentation and outcome of autosomal dominant polycystic kidney disease in Nigeria. African health sciences. 2018 Sep:18(3):671-680. doi: 10.4314/ahs.v18i3.25. Epub
[PubMed PMID: 30603000]
[22]
Müller RU, Benzing T. Management of autosomal-dominant polycystic kidney disease-state-of-the-art. Clinical kidney journal. 2018 Dec:11(Suppl 1):i2-i13. doi: 10.1093/ckj/sfy103. Epub 2018 Dec 17
[PubMed PMID: 30581561]
[23]
Smith KA, Thompson AM, Baron DA, Broadbent ST, Lundstrom GH, Perrone RD. Addressing the Need for Clinical Trial End Points in Autosomal Dominant Polycystic Kidney Disease: A Report From the Polycystic Kidney Disease Outcomes Consortium (PKDOC). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2019 Apr:73(4):533-541. doi: 10.1053/j.ajkd.2018.11.001. Epub 2018 Dec 29
[PubMed PMID: 30600104]
[24]
Weimbs T, Shillingford JM, Torres J, Kruger SL, Bourgeois BC. Emerging targeted strategies for the treatment of autosomal dominant polycystic kidney disease. Clinical kidney journal. 2018 Dec:11(Suppl 1):i27-i38. doi: 10.1093/ckj/sfy089. Epub 2018 Dec 17
[PubMed PMID: 30581563]
[25]
Torres VE. Pro: Tolvaptan delays the progression of autosomal dominant polycystic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2019 Jan 1:34(1):30-34. doi: 10.1093/ndt/gfy297. Epub
[PubMed PMID: 30312438]
[26]
Raina R, Houry A, Rath P, Mangat G, Pandher D, Islam M, Khattab AG, Kalout JK, Bagga S. Clinical Utility and Tolerability of Tolvaptan in the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Drug, healthcare and patient safety. 2022:14():147-159. doi: 10.2147/DHPS.S338050. Epub 2022 Sep 8
[PubMed PMID: 36105663]
[27]
Müller RU, Messchendorp AL, Birn H, Capasso G, Cornec-Le Gall E, Devuyst O, van Eerde A, Guirchoun P, Harris T, Hoorn EJ, Knoers NVAM, Korst U, Mekahli D, Le Meur Y, Nijenhuis T, Ong ACM, Sayer JA, Schaefer F, Servais A, Tesar V, Torra R, Walsh SB, Gansevoort RT. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2022 Apr 25:37(5):825-839. doi: 10.1093/ndt/gfab312. Epub
[PubMed PMID: 35134221]
Level 3 (low-level) evidence
[28]
Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, Torres VE. A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. Journal of the American Society of Nephrology : JASN. 2018 Oct:29(10):2458-2470. doi: 10.1681/ASN.2018060590. Epub 2018 Sep 18
[PubMed PMID: 30228150]
[29]
Torres VE, Abebe KZ, Chapman AB, Schrier RW, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Moore CG, Perrone RD, HALT-PKD Trial Investigators. Angiotensin blockade in late autosomal dominant polycystic kidney disease. The New England journal of medicine. 2014 Dec 11:371(24):2267-76. doi: 10.1056/NEJMoa1402686. Epub 2014 Nov 15
[PubMed PMID: 25399731]
[30]
Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Bae KT, Moore CG, Chapman AB, HALT-PKD Trial Investigators. Blood pressure in early autosomal dominant polycystic kidney disease. The New England journal of medicine. 2014 Dec 11:371(24):2255-66. doi: 10.1056/NEJMoa1402685. Epub 2014 Nov 15
[PubMed PMID: 25399733]
[31]
Shoaf SE, Ouyang J, Sergeyeva O, Estilo A, Li H, Leung D. A Post Hoc Analysis of Statin Use in Tolvaptan Autosomal Dominant Polycystic Kidney Disease Pivotal Trials. Clinical journal of the American Society of Nephrology : CJASN. 2020 May 7:15(5):643-650. doi: 10.2215/CJN.08170719. Epub 2020 Apr 2
[PubMed PMID: 32241780]
[32]
Xue C, Zhang LM, Zhou C, Mei CL, Yu SQ. Effect of Statins on Renal Function and Total Kidney Volume in Autosomal Dominant Polycystic Kidney Disease. Kidney diseases (Basel, Switzerland). 2020 Nov:6(6):407-413. doi: 10.1159/000509087. Epub 2020 Jul 29
[PubMed PMID: 33313061]
[33]
Sung PH, Chiang HJ, Lee MS, Chiang JY, Yip HK, Yang YH. Combined renin-angiotensin-aldosterone system blockade and statin therapy effectively reduces the risk of cerebrovascular accident in autosomal dominant polycystic kidney disease: a nationwide population-based cohort study. Oncotarget. 2017 Sep 22:8(37):61570-61582. doi: 10.18632/oncotarget.18636. Epub 2017 Jun 27
[PubMed PMID: 28977886]
[34]
Lin CH, Chao CT, Wu MY, Lo WC, Lin TC, Wu MS. Use of mammalian target of rapamycin inhibitors in patient with autosomal dominant polycystic kidney disease: an updated meta-analysis. International urology and nephrology. 2019 Nov:51(11):2015-2025. doi: 10.1007/s11255-019-02292-1. Epub 2019 Oct 1
[PubMed PMID: 31578673]
Level 1 (high-level) evidence
[35]
Messchendorp AL, Spithoven EM, Casteleijn NF, Dam WA, van den Born J, Tonnis WF, Gaillard CAJM, Meijer E, DIPAK Consortium. Association of plasma somatostatin with disease severity and progression in patients with autosomal dominant polycystic kidney disease. BMC nephrology. 2018 Dec 19:19(1):368. doi: 10.1186/s12882-018-1176-y. Epub 2018 Dec 19
[PubMed PMID: 30567514]
[36]
Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nature reviews. Disease primers. 2018 Dec 6:4(1):50. doi: 10.1038/s41572-018-0047-y. Epub 2018 Dec 6
[PubMed PMID: 30523303]
[37]
Perrone RD, Abebe KZ, Watnick TJ, Althouse AD, Hallows KR, Lalama CM, Miskulin DC, Seliger SL, Tao C, Harris PC, Bae KT. Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD). Kidney international. 2021 Sep:100(3):684-696. doi: 10.1016/j.kint.2021.06.013. Epub 2021 Jun 27
[PubMed PMID: 34186056]
Level 1 (high-level) evidence
[39]
Emre H, Turgay A, Ali A, Murat B, Ozgür Y, Cankon G. 'Stepped procedure' in laparoscopic cyst decortication during the learning period of laparoscopic surgery: Detailed evaluation of initial experiences. Journal of minimal access surgery. 2010 Apr:6(2):37-41. doi: 10.4103/0972-9941.65162. Epub
[PubMed PMID: 20814509]
[40]
Zhu XH, Zhang X, Han XW, Zhang P, Wang SY, Li YS, Li G, Chen YH. A controlled clinical study of a two-trocar mini-laparoscopic technique versus the standard laparoscopic technique in treatment of adult renal cysts. Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques. 2021 Dec:16(4):728-735. doi: 10.5114/wiitm.2021.104173. Epub 2021 Mar 5
[PubMed PMID: 34950269]
[41]
Hong Y, Chen X, Wu M, Xi H, Hu J. Percutaneous vs Laparoscopic Treatment for Simple Renal Cysts: A Meta-analysis. Journal of endourology. 2021 Dec:35(12):1793-1800. doi: 10.1089/end.2021.0264. Epub
[PubMed PMID: 34036798]
Level 1 (high-level) evidence