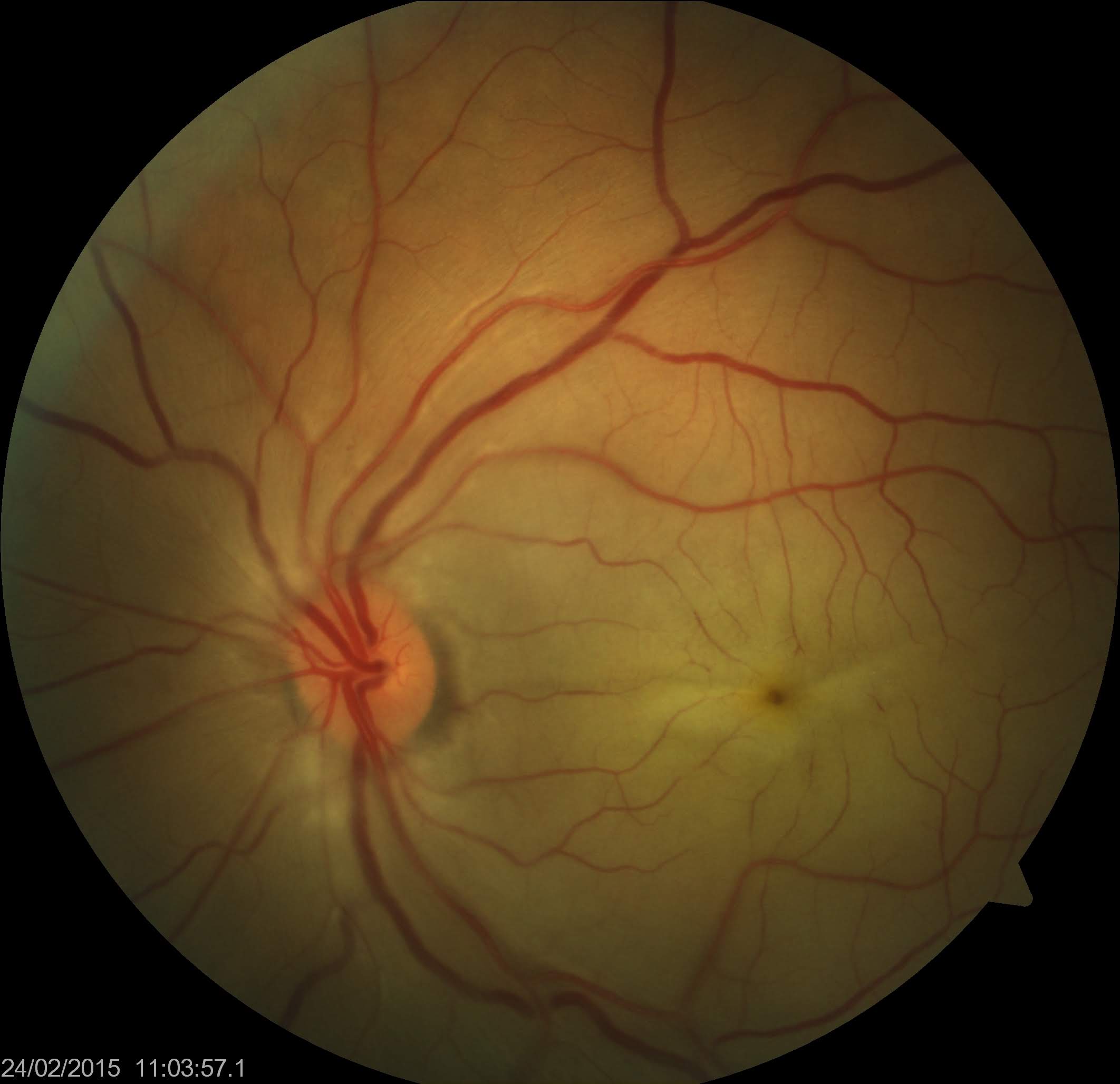
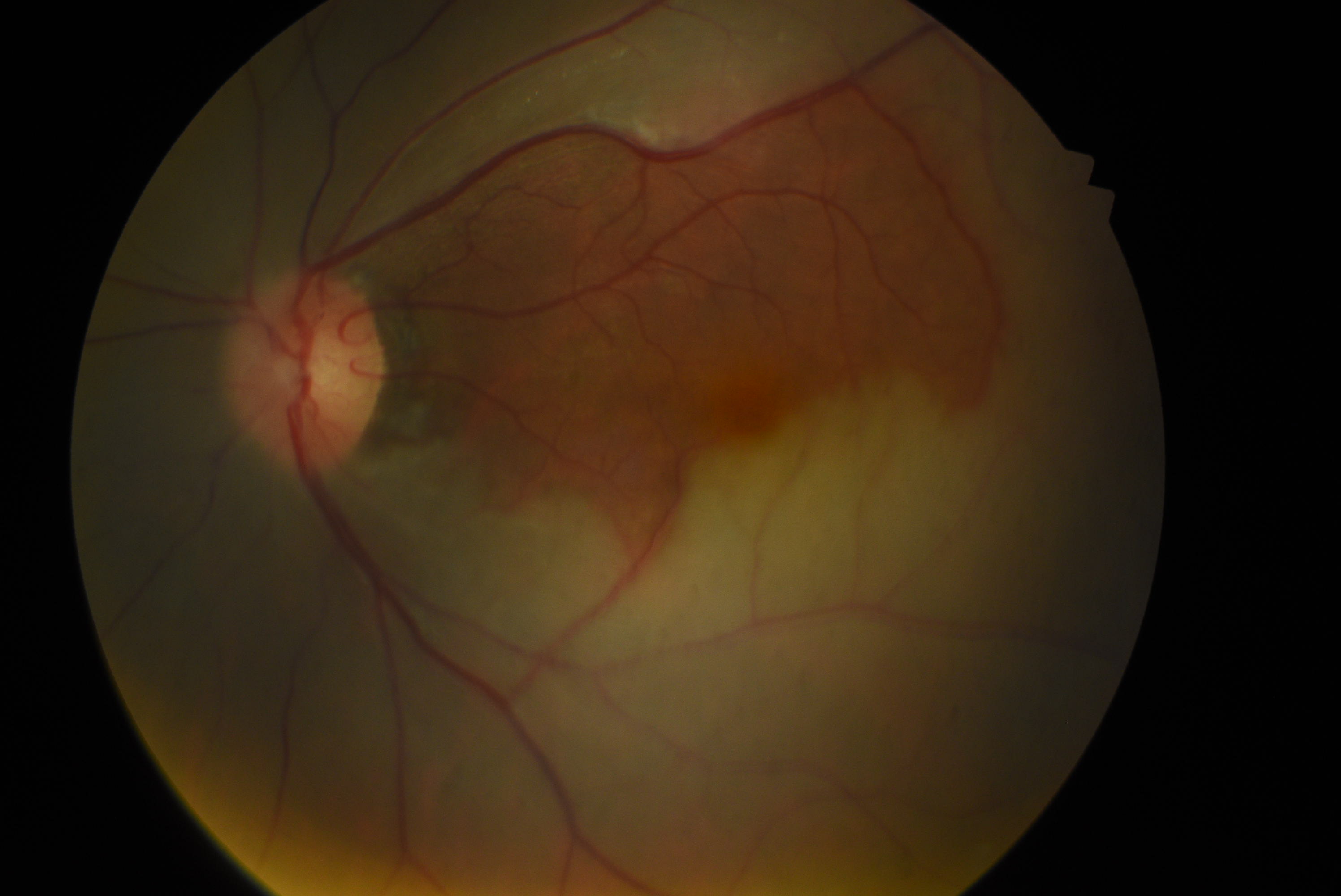
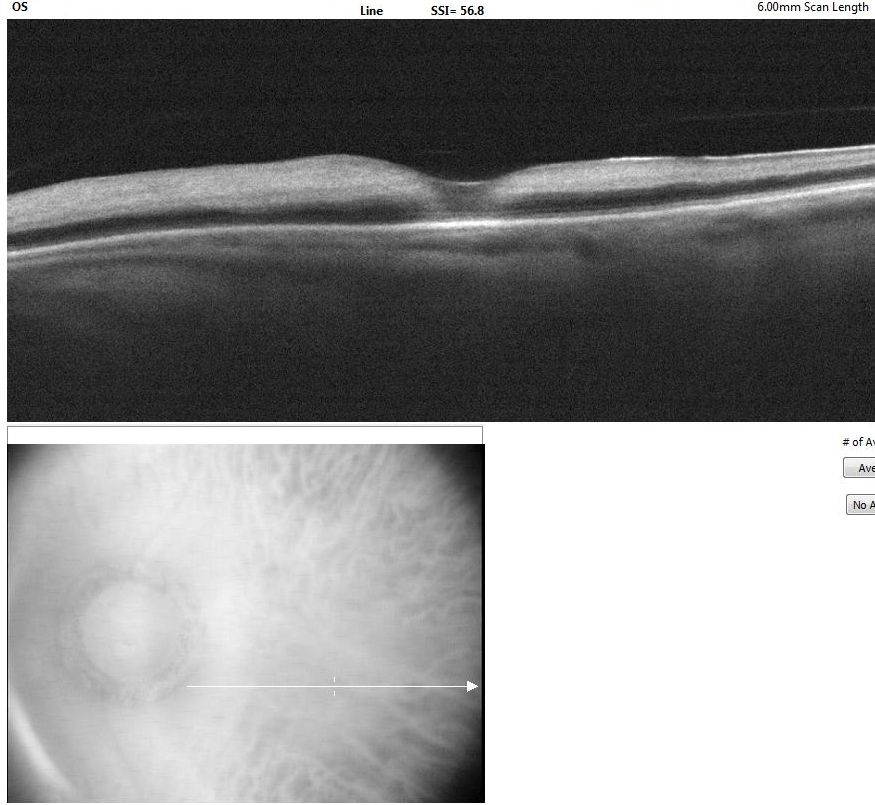
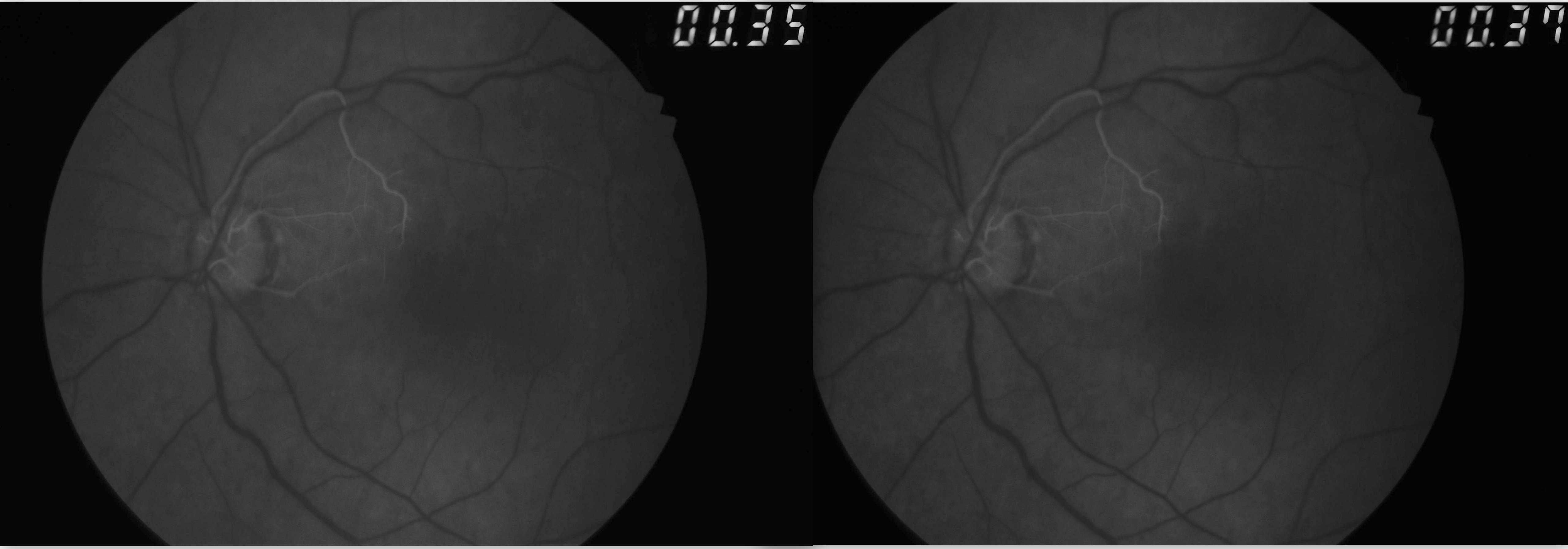
[1]
Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Current treatment options in neurology. 2013 Feb:15(1):63-77. doi: 10.1007/s11940-012-0202-9. Epub
[PubMed PMID: 23070637]
[2]
Hayreh SS. Central retinal artery occlusion. Indian journal of ophthalmology. 2018 Dec:66(12):1684-1694. doi: 10.4103/ijo.IJO_1446_18. Epub
[PubMed PMID: 30451166]
[3]
Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (London, England). 2013 Jun:27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8
[PubMed PMID: 23470793]
[4]
Avery MB, Magal I, Kherani A, Mitha AP. Risk of Stroke in Patients With Ocular Arterial Occlusive Disorders: A Retrospective Canadian Study. Journal of the American Heart Association. 2019 Feb 5:8(3):e010509. doi: 10.1161/JAHA.118.010509. Epub
[PubMed PMID: 30712440]
Level 2 (mid-level) evidence
[5]
Mir TA, Arham AZ, Fang W, Alqahtani F, Alkhouli M, Gallo J, Hinkle DM. Acute Vascular Ischemic Events in Patients With Central Retinal Artery Occlusion in the United States: A Nationwide Study 2003-2014. American journal of ophthalmology. 2019 Apr:200():179-186. doi: 10.1016/j.ajo.2019.01.009. Epub 2019 Jan 26
[PubMed PMID: 30689989]
[6]
Zhou Y, Zhu W, Wang C. Relationship between retinal vascular occlusions and incident cerebrovascular diseases: A systematic review and meta-analysis. Medicine. 2016 Jun:95(26):e4075. doi: 10.1097/MD.0000000000004075. Epub
[PubMed PMID: 27368050]
Level 1 (high-level) evidence
[7]
Dattilo M, Biousse V, Newman NJ. Update on the Management of Central Retinal Artery Occlusion. Neurologic clinics. 2017 Feb:35(1):83-100. doi: 10.1016/j.ncl.2016.08.013. Epub
[PubMed PMID: 27886897]
[8]
Mac Grory B, Schrag M, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, Sobrin L, Tjoumakaris SI, Weyand CM, Yaghi S, American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; and Council on Peripheral Vascular Disease. Management of Central Retinal Artery Occlusion: A Scientific Statement From the American Heart Association. Stroke. 2021 Jun:52(6):e282-e294. doi: 10.1161/STR.0000000000000366. Epub 2021 Mar 8
[PubMed PMID: 33677974]
[10]
Tripathy K, Mazumdar S, Sarma B. Central retinal arterial occlusion in a patient with pyoderma gangrenosum. Indian journal of ophthalmology. 2018 Jul:66(7):1019-1021. doi: 10.4103/ijo.IJO_1229_17. Epub
[PubMed PMID: 29941761]
[11]
Liu W, Bai D, Kou L. Progress in central retinal artery occlusion: a narrative review. The Journal of international medical research. 2023 Sep:51(9):3000605231198388. doi: 10.1177/03000605231198388. Epub
[PubMed PMID: 37712755]
Level 3 (low-level) evidence
[12]
Madike R, Cugati S, Chen C. A review of the management of central retinal artery occlusion. Taiwan journal of ophthalmology. 2022 Jul-Sep:12(3):273-281. doi: 10.4103/2211-5056.353126. Epub 2022 Aug 18
[PubMed PMID: 36248088]
[13]
Babikian V, Wijman CA, Koleini B, Malik SN, Goyal N, Matjucha IC. Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovascular diseases (Basel, Switzerland). 2001 Aug:12(2):108-13
[PubMed PMID: 11490104]
[14]
Ahuja RM, Chaturvedi S, Eliott D, Joshi N, Puklin JE, Abrams GW. Mechanisms of retinal arterial occlusive disease in African American and Caucasian patients. Stroke. 1999 Aug:30(8):1506-9
[PubMed PMID: 10436091]
[15]
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern®. Ophthalmology. 2020 Feb:127(2):P259-P287. doi: 10.1016/j.ophtha.2019.09.028. Epub 2019 Sep 25
[PubMed PMID: 31757501]
[17]
Cheung N, Lim L, Wang JJ, Islam FM, Mitchell P, Saw SM, Aung T, Wong TY. Prevalence and risk factors of retinal arteriolar emboli: the Singapore Malay Eye Study. American journal of ophthalmology. 2008 Oct:146(4):620-4. doi: 10.1016/j.ajo.2008.05.033. Epub 2008 Jul 21
[PubMed PMID: 18639861]
[18]
O'Donnell BA, Mitchell P. The clinical features and associations of retinal emboli. Australian and New Zealand journal of ophthalmology. 1992 Feb:20(1):11-7
[PubMed PMID: 1599661]
[19]
Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal emboli in an older population. Stroke. 2006 Mar:37(3):908-10
[PubMed PMID: 16439697]
[20]
Mitchell P, Wang JJ, Li W, Leeder SR, Smith W. Prevalence of asymptomatic retinal emboli in an Australian urban community. Stroke. 1997 Jan:28(1):63-6
[PubMed PMID: 8996490]
[21]
Hadley G, Earnshaw JJ, Stratton I, Sykes J, Scanlon PH. A potential pathway for managing diabetic patients with arterial emboli detected by retinal screening. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2011 Aug:42(2):153-7. doi: 10.1016/j.ejvs.2011.04.031. Epub 2011 May 25
[PubMed PMID: 21616692]
[22]
Ramakrishna G, Malouf JF, Younge BR, Connolly HM, Miller FA. Calcific retinal embolism as an indicator of severe unrecognised cardiovascular disease. Heart (British Cardiac Society). 2005 Sep:91(9):1154-7
[PubMed PMID: 16103545]
[23]
Cho KH, Ahn SJ, Cho JH, Jung C, Han MK, Park SJ, Park KH, Woo SJ. The Characteristics of Retinal Emboli and its Association With Vascular Reperfusion in Retinal Artery Occlusion. Investigative ophthalmology & visual science. 2016 Sep 1:57(11):4589-98. doi: 10.1167/iovs.16-19887. Epub
[PubMed PMID: 27598864]
[25]
Terao R, Fujino R, Ahmed T. Risk Factors and Treatment Strategy for Retinal Vascular Occlusive Diseases. Journal of clinical medicine. 2022 Oct 27:11(21):. doi: 10.3390/jcm11216340. Epub 2022 Oct 27
[PubMed PMID: 36362567]
[26]
Asensio-Sánchez VM. Central retinal artery occlusion following facial injection of hyaluronic acid. Archivos de la Sociedad Espanola de Oftalmologia. 2023 Jul:98(7):410-412. doi: 10.1016/j.oftale.2023.05.008. Epub 2023 May 27
[PubMed PMID: 37247664]
[27]
Gupta V, Luthra S, Shrinkhal N, Sinha S. Takayasu's arteritis: a unique ophthalmic presentation with CRAO and BRVO. BMJ case reports. 2019 Aug 15:12(8):. doi: 10.1136/bcr-2018-228909. Epub 2019 Aug 15
[PubMed PMID: 31420422]
Level 3 (low-level) evidence
[28]
Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. American journal of ophthalmology. 1997 Mar:123(3):285-96
[PubMed PMID: 9063237]
[29]
Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. American journal of ophthalmology. 2005 Sep:140(3):376-91
[PubMed PMID: 16138997]
[30]
Kido A, Tamura H, Ikeda HO, Miyake M, Hiragi S, Tsujikawa A. Nationwide incidence of central retinal artery occlusion in Japan: an exploratory descriptive study using the National Database of Health Insurance Claims (2011-2015). BMJ open. 2020 Sep 24:10(9):e041104. doi: 10.1136/bmjopen-2020-041104. Epub 2020 Sep 24
[PubMed PMID: 32973068]
[31]
Pick J, Nickels S, Saalmann F, Finger RP, Schuster AK. Incidence of retinal artery occlusion in Germany. Acta ophthalmologica. 2020 Aug:98(5):e656-e657. doi: 10.1111/aos.14369. Epub 2020 Feb 6
[PubMed PMID: 32026572]
[32]
Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology. 2014 Oct:121(10):1933-8. doi: 10.1016/j.ophtha.2014.04.029. Epub 2014 Jun 7
[PubMed PMID: 24913283]
[33]
Ivanisević M, Karelović D. The incidence of central retinal artery occlusion in the district of Split, Croatia. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2001 May-Jun:215(3):245-6
[PubMed PMID: 11340401]
[34]
Abbati G, Fazi C, Fortunato P, Trapani S. Central retinal artery occlusion in a young child affected by COVID-19: a first case report. BMC pediatrics. 2023 Sep 13:23(1):462. doi: 10.1186/s12887-023-04276-8. Epub 2023 Sep 13
[PubMed PMID: 37704960]
Level 3 (low-level) evidence
[35]
Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. American journal of ophthalmology. 2011 Nov:152(5):820-3.e2. doi: 10.1016/j.ajo.2011.05.005. Epub 2011 Jul 27
[PubMed PMID: 21794842]
[36]
Agarwal N, Gala NB, Karimi RJ, Turbin RE, Gandhi CD, Prestigiacomo CJ. Current endovascular treatment options for central retinal arterial occlusion: a review. Neurosurgical focus. 2014 Jan:36(1):E7. doi: 10.3171/2013.11.FOCUS13331. Epub
[PubMed PMID: 24380484]
[37]
Grzybowski AE, Mimier MK. Evaluation of the Association between the Risk of Central Retinal Artery Occlusion and the Concentration of Environmental Air Pollutants. Journal of clinical medicine. 2019 Feb 7:8(2):. doi: 10.3390/jcm8020206. Epub 2019 Feb 7
[PubMed PMID: 30736427]
[38]
Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002 Aug:33(8):1963-7
[PubMed PMID: 12154246]
[39]
Schumacher M, Schmidt D, Jurklies B, Gall C, Wanke I, Schmoor C, Maier-Lenz H, Solymosi L, Brueckmann H, Neubauer AS, Wolf A, Feltgen N, EAGLE-Study Group. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010 Jul:117(7):1367-75.e1. doi: 10.1016/j.ophtha.2010.03.061. Epub
[PubMed PMID: 20609991]
Level 1 (high-level) evidence
[40]
Lavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke Risk and Risk Factors in Patients With Central Retinal Artery Occlusion. American journal of ophthalmology. 2019 Apr:200():271-272. doi: 10.1016/j.ajo.2019.01.021. Epub 2019 Feb 28
[PubMed PMID: 30827486]
[42]
Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Experimental eye research. 2004 Mar:78(3):723-36
[PubMed PMID: 15106952]
[43]
Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion - rethinking retinal survival time. BMC ophthalmology. 2018 Apr 18:18(1):101. doi: 10.1186/s12886-018-0768-4. Epub 2018 Apr 18
[PubMed PMID: 29669523]
[44]
Chronopoulos A, Schutz JS. Central retinal artery occlusion-A new, provisional treatment approach. Survey of ophthalmology. 2019 Jul-Aug:64(4):443-451. doi: 10.1016/j.survophthal.2019.01.011. Epub 2019 Jan 30
[PubMed PMID: 30707925]
Level 3 (low-level) evidence
[46]
Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, Santiago AR. Neuroprotective Strategies for Retinal Ganglion Cell Degeneration: Current Status and Challenges Ahead. International journal of molecular sciences. 2020 Mar 25:21(7):. doi: 10.3390/ijms21072262. Epub 2020 Mar 25
[PubMed PMID: 32218163]
[47]
Sun Y, Smith LEH. Retinal Vasculature in Development and Diseases. Annual review of vision science. 2018 Sep 15:4():101-122. doi: 10.1146/annurev-vision-091517-034018. Epub
[PubMed PMID: 30222533]
[49]
Merriam JC, Casper DS. The entry point of the central retinal artery into the outer meningeal sheath of the optic nerve. Clinical anatomy (New York, N.Y.). 2021 May:34(4):605-608. doi: 10.1002/ca.23637. Epub 2020 Jul 1
[PubMed PMID: 32530060]
[51]
Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982 Jan:89(1):14-9
[PubMed PMID: 7070767]
[53]
Carranza-Casas M, Aceves-Velazquez JE, Cano-Hidalgo R, Graue-Wiechers F. Partial Central Retinal Artery Occlusion: An Underrecognized Entity. International medical case reports journal. 2020:13():637-642. doi: 10.2147/IMCRJ.S274409. Epub 2020 Nov 26
[PubMed PMID: 33273866]
Level 3 (low-level) evidence
[54]
Gong H, Wu B, Xie S. Visual acuity assessment of central retinal artery occlusion patients with or without paracentral acute middle maculopathy via OCT-A. BMC ophthalmology. 2023 Oct 13:23(1):412. doi: 10.1186/s12886-023-03151-5. Epub 2023 Oct 13
[PubMed PMID: 37833625]
[55]
Bakhoum MF, Freund KB, Dolz-Marco R, Leong BCS, Baumal CR, Duker JS, Sarraf D. Paracentral Acute Middle Maculopathy and the Ischemic Cascade Associated With Retinal Vascular Occlusion. American journal of ophthalmology. 2018 Nov:195():143-153. doi: 10.1016/j.ajo.2018.07.031. Epub 2018 Aug 3
[PubMed PMID: 30081014]
[56]
Wenzel DA, Kromer R, Poli S, Steinhorst NA, Casagrande MK, Spitzer MS, Schultheiss M. Optical coherence tomography-based determination of ischaemia onset - the temporal dynamics of retinal thickness increase in acute central retinal artery occlusion. Acta ophthalmologica. 2021 Mar:99(2):e247-e252. doi: 10.1111/aos.14563. Epub 2020 Aug 6
[PubMed PMID: 32767551]
[57]
Fan W, Huang Y, Zhao Y, Yuan R. Central retinal artery occlusion without cherry-red spots. BMC ophthalmology. 2023 Oct 25:23(1):434. doi: 10.1186/s12886-023-03176-w. Epub 2023 Oct 25
[PubMed PMID: 37880636]
[60]
Duker JS, Sivalingam A, Brown GC, Reber R. A prospective study of acute central retinal artery obstruction. The incidence of secondary ocular neovascularization. Archives of ophthalmology (Chicago, Ill. : 1960). 1991 Mar:109(3):339-42
[PubMed PMID: 1706177]
[61]
Tripathy K, Chawla R. Extensive commotio retinae involving peripheral retina. The National medical journal of India. 2017 Jul-Aug:30(4):242. doi: 10.4103/0970-258X.218686. Epub
[PubMed PMID: 29162764]
[62]
Ahmed NR, Tripathy K, Kumar V, Gogia V. Choroidal coloboma in a case of tay-sachs disease. Case reports in ophthalmological medicine. 2014:2014():760746. doi: 10.1155/2014/760746. Epub 2014 Sep 10
[PubMed PMID: 25295204]
Level 3 (low-level) evidence
[63]
Kruszewski AM, Tamhankar MA. Ophthalmic Manifestations of Giant Cell Arteritis. International ophthalmology clinics. 2023 Apr 1:63(2):13-23. doi: 10.1097/IIO.0000000000000465. Epub 2023 Mar 23
[PubMed PMID: 36963824]
[64]
Hayreh SS. Anterior ischaemic optic neuropathy. II. Fundus on ophthalmoscopy and fluorescein angiography. The British journal of ophthalmology. 1974 Dec:58(12):964-80
[PubMed PMID: 4376416]
[65]
Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. American journal of ophthalmology. 1998 Apr:125(4):509-20
[PubMed PMID: 9559737]
[67]
Liang S, Chen Q, Hu C, Chen M. Association of Paracentral Acute Middle Maculopathy with Visual Prognosis in Retinal Artery Occlusion: A Retrospective Cohort Study. Journal of ophthalmology. 2022:2022():9404973. doi: 10.1155/2022/9404973. Epub 2022 May 21
[PubMed PMID: 35637681]
Level 2 (mid-level) evidence
[68]
Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina (Philadelphia, Pa.). 2007 Mar:27(3):276-89
[PubMed PMID: 17460582]
[69]
Lee WA, Liao IC. Giant cell arteritis presenting as central retinal artery occlusion. QJM : monthly journal of the Association of Physicians. 2022 Jan 21:115(1):32-33. doi: 10.1093/qjmed/hcab196. Epub
[PubMed PMID: 34264347]
[71]
Achkar AA, Lie JT, Hunder GG, O'Fallon WM, Gabriel SE. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Annals of internal medicine. 1994 Jun 15:120(12):987-92
[PubMed PMID: 8185147]
[72]
Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, Wachter R, Schmoor C, Schumacher M, Junker B, Pielen A, European Assessment Group for Lysis in the Eye. Cardiovascular Risk Factors in Central Retinal Artery Occlusion: Results of a Prospective and Standardized Medical Examination. Ophthalmology. 2015 Sep:122(9):1881-8. doi: 10.1016/j.ophtha.2015.05.044. Epub 2015 Jul 21
[PubMed PMID: 26231133]
[73]
Chen CS, Lee AW, Campbell B, Lee T, Paine M, Fraser C, Grigg J, Markus R. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion: report from a randomized, controlled trial. Stroke. 2011 Aug:42(8):2229-34. doi: 10.1161/STROKEAHA.111.613653. Epub 2011 Jul 14
[PubMed PMID: 21757667]
Level 1 (high-level) evidence
[74]
Beatty S, Au Eong KG. Acute occlusion of the retinal arteries: current concepts and recent advances in diagnosis and management. Journal of accident & emergency medicine. 2000 Sep:17(5):324-9
[PubMed PMID: 11005400]
Level 3 (low-level) evidence
[75]
Ffytche TJ. A rationalization of treatment of central retinal artery occlusion. Transactions of the ophthalmological societies of the United Kingdom. 1974 Jul:94(2):468-79
[PubMed PMID: 4619853]
[76]
Fieß A, Cal Ö, Kehrein S, Halstenberg S, Frisch I, Steinhorst UH. Anterior chamber paracentesis after central retinal artery occlusion: a tenable therapy? BMC ophthalmology. 2014 Mar 10:14():28. doi: 10.1186/1471-2415-14-28. Epub 2014 Mar 10
[PubMed PMID: 24612658]
[77]
Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995 Dec:102(12):2029-34; discussion 2034-5
[PubMed PMID: 9098313]
[78]
Incandela L, Cesarone MR, Belcaro G, Steigerwalt R, De Sanctis MT, Nicolaides AN, Griffin M, Geroulakos G, Ramaswami G. Treatment of vascular retinal disease with pentoxifylline: a controlled, randomized trial. Angiology. 2002 Jan-Feb:53 Suppl 1():S31-4
[PubMed PMID: 11865833]
Level 1 (high-level) evidence
[79]
Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. The Cochrane database of systematic reviews. 2009 Jan 21:2009(1):CD001989. doi: 10.1002/14651858.CD001989.pub2. Epub 2009 Jan 21
[PubMed PMID: 19160204]
Level 1 (high-level) evidence
[80]
Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. American journal of ophthalmology. 1999 Dec:128(6):733-8
[PubMed PMID: 10612510]
Level 1 (high-level) evidence
[81]
Nedelmann M, Graef M, Weinand F, Wassill KH, Kaps M, Lorenz B, Tanislav C. Retrobulbar Spot Sign Predicts Thrombolytic Treatment Effects and Etiology in Central Retinal Artery Occlusion. Stroke. 2015 Aug:46(8):2322-4. doi: 10.1161/STROKEAHA.115.009839. Epub 2015 Jun 25
[PubMed PMID: 26111890]
[82]
Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous Fibrinolytic Therapy in Central Retinal Artery Occlusion: A Patient-Level Meta-analysis. JAMA neurology. 2015 Oct:72(10):1148-54. doi: 10.1001/jamaneurol.2015.1578. Epub
[PubMed PMID: 26258861]
Level 1 (high-level) evidence
[83]
Butler FK Jr, Hagan C, Murphy-Lavoie H. Hyperbaric oxygen therapy and the eye. Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society, Inc. 2008 Sep-Oct:35(5):333-87
[PubMed PMID: 19024664]
[84]
Mehboob MA, Khan A, Mukhtar A. Efficacy of YAG Laser Embolysis in Retinal Artery Occlusion. Pakistan journal of medical sciences. 2021 Jan-Feb:37(1):71-75. doi: 10.12669/pjms.37.1.3196. Epub
[PubMed PMID: 33437253]
[85]
Man V, Hecht I, Talitman M, Hilely A, Midlij M, Burgansky-Eliash Z, Achiron A. Treatment of retinal artery occlusion using transluminal Nd:YAG laser: a systematic review and meta-analysis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2017 Oct:255(10):1869-1877. doi: 10.1007/s00417-017-3777-8. Epub 2017 Aug 19
[PubMed PMID: 28823062]
Level 1 (high-level) evidence
[86]
Youn TS, Lavin P, Patrylo M, Schindler J, Kirshner H, Greer DM, Schrag M. Current treatment of central retinal artery occlusion: a national survey. Journal of neurology. 2018 Feb:265(2):330-335. doi: 10.1007/s00415-017-8702-x. Epub 2017 Dec 13
[PubMed PMID: 29236169]
Level 3 (low-level) evidence
[87]
García-Arumí J, Martinez-Castillo V, Boixadera A, Fonollosa A, Corcostegui B. Surgical embolus removal in retinal artery occlusion. The British journal of ophthalmology. 2006 Oct:90(10):1252-5
[PubMed PMID: 16854826]
[88]
Tang WM, Topping TM. Vitreous surgery for central retinal artery occlusion. Archives of ophthalmology (Chicago, Ill. : 1960). 2000 Nov:118(11):1586-7
[PubMed PMID: 11074821]
[89]
Hayreh SS, Ocular vascular occlusive disorders: natural history of visual outcome. Progress in retinal and eye research. 2014 Jul;
[PubMed PMID: 24769221]
[90]
Chodnicki KD, Pulido JS, Hodge DO, Klaas JP, Chen JJ. Stroke Risk Before and After Central Retinal Artery Occlusion in a US Cohort. Mayo Clinic proceedings. 2019 Feb:94(2):236-241. doi: 10.1016/j.mayocp.2018.10.018. Epub
[PubMed PMID: 30711121]
[91]
Shaikh IS, Elsamna ST, Zarbin MA, Bhagat N. Assessing the risk of stroke development following retinal artery occlusion. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2020 Sep:29(9):105002. doi: 10.1016/j.jstrokecerebrovasdis.2020.105002. Epub 2020 Jun 15
[PubMed PMID: 32807420]
[92]
Hwang DD, Lee KE, Kim Y, Kim MS, Rim TH, Kim M, Kim H, Kyoung DS, Park JI. Incidence of Retinal Artery Occlusion and Related Mortality in Korea, 2005 to 2018. JAMA network open. 2023 Mar 1:6(3):e233068. doi: 10.1001/jamanetworkopen.2023.3068. Epub 2023 Mar 1
[PubMed PMID: 36897587]
[93]
Duker JS, Brown GC. The efficacy of panretinal photocoagulation for neovascularization of the iris after central retinal artery obstruction. Ophthalmology. 1989 Jan:96(1):92-5
[PubMed PMID: 2465523]
[94]
Hayreh SS. Giant cell arteritis: Its ophthalmic manifestations. Indian journal of ophthalmology. 2021 Feb:69(2):227-235. doi: 10.4103/ijo.IJO_1681_20. Epub
[PubMed PMID: 33463564]
[95]
Rippe JM. Lifestyle Strategies for Risk Factor Reduction, Prevention, and Treatment of Cardiovascular Disease. American journal of lifestyle medicine. 2019 Mar-Apr:13(2):204-212. doi: 10.1177/1559827618812395. Epub 2018 Dec 2
[PubMed PMID: 30800027]
[96]
Olson EA, Lentz K. Central Retinal Artery Occlusion: A Literature Review and the Rationale for Hyperbaric Oxygen Therapy. Missouri medicine. 2016 Jan-Feb:113(1):53-7
[PubMed PMID: 27039492]