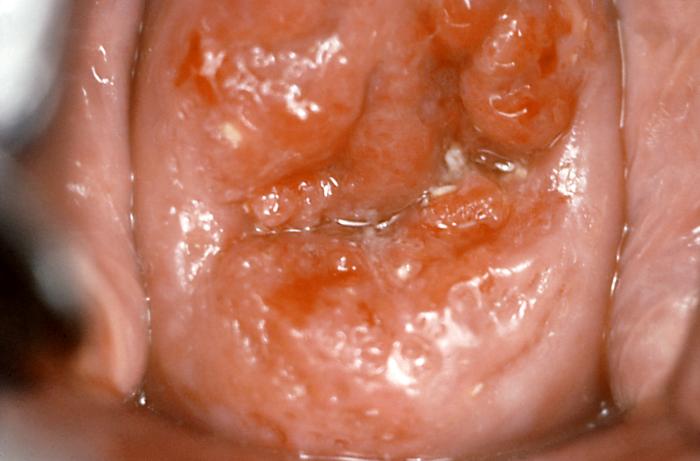
[1]
Brisson M, Drolet M. Global elimination of cervical cancer as a public health problem. The Lancet. Oncology. 2019 Mar:20(3):319-321. doi: 10.1016/S1470-2045(19)30072-5. Epub 2019 Feb 19
[PubMed PMID: 30795952]
[2]
Pimple SA, Mishra GA. Global strategies for cervical cancer prevention and screening. Minerva ginecologica. 2019 Aug:71(4):313-320. doi: 10.23736/S0026-4784.19.04397-1. Epub 2019 Feb 22
[PubMed PMID: 30808155]
[3]
. Cervical Cancer Screening Every 5 Years OK. Cancer discovery. 2018 Oct:8(10):1204. doi: 10.1158/2159-8290.CD-NB2018-118. Epub 2018 Sep 11
[PubMed PMID: 30206109]
[4]
Farghaly H, Bourgeois D, Houser PM, Padmanabhan V, Lage JM, Hoda RS. Routine vaginal Pap test is not useful in women status-post hysterectomy for benign disease. Diagnostic cytopathology. 2006 Sep:34(9):640-3
[PubMed PMID: 16900480]
[5]
Foran C, Brennan A. Prevention and early detection of cervical cancer in the UK. British journal of nursing (Mark Allen Publishing). 2015 May 28-Jun 10:24(10):S22-4, S26, S28-9. doi: 10.12968/bjon.2015.24.Sup10.S22. Epub
[PubMed PMID: 26018178]
[6]
Pierre-Victor D, Stephens DP, Omondi A, Clarke R, Jean-Baptiste N, Madhivanan P. Barriers to HPV Vaccination Among Unvaccinated, Haitian American College Women. Health equity. 2018:2(1):90-97. doi: 10.1089/heq.2017.0028. Epub 2018 Jun 1
[PubMed PMID: 29904749]
[7]
Manini I, Montomoli E. Epidemiology and prevention of Human Papillomavirus. Annali di igiene : medicina preventiva e di comunita. 2018 Jul-Aug:30(4 Supple 1):28-32. doi: 10.7416/ai.2018.2231. Epub
[PubMed PMID: 30062377]
[8]
Ghosh I, Mandal R, Kundu P, Biswas J. Association of Genital Infections Other Than Human Papillomavirus with Pre-Invasive and Invasive Cervical Neoplasia. Journal of clinical and diagnostic research : JCDR. 2016 Feb:10(2):XE01-XE06. doi: 10.7860/JCDR/2016/15305.7173. Epub 2016 Feb 1
[PubMed PMID: 27042571]
[9]
Habtemariam LW, Zewde ET, Simegn GL. Cervix Type and Cervical Cancer Classification System Using Deep Learning Techniques. Medical devices (Auckland, N.Z.). 2022:15():163-176. doi: 10.2147/MDER.S366303. Epub 2022 Jun 16
[PubMed PMID: 35734419]
[10]
Kuhn L, Denny L. The time is now to implement HPV testing for primary screening in low resource settings. Preventive medicine. 2017 May:98():42-44. doi: 10.1016/j.ypmed.2016.12.030. Epub 2017 Feb 6
[PubMed PMID: 28279263]
[11]
Rauh-Hain JA, Melamed A, Schaps D, Bregar AJ, Spencer R, Schorge JO, Rice LW, Del Carmen MG. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecologic oncology. 2018 Apr:149(1):4-11. doi: 10.1016/j.ygyno.2017.12.006. Epub
[PubMed PMID: 29605048]
Level 2 (mid-level) evidence
[12]
Wang X, Huang X, Zhang Y. Involvement of Human Papillomaviruses in Cervical Cancer. Frontiers in microbiology. 2018:9():2896. doi: 10.3389/fmicb.2018.02896. Epub 2018 Nov 28
[PubMed PMID: 30546351]
[13]
Romero-Masters JC, Lambert PF, Munger K. Molecular Mechanisms of MmuPV1 E6 and E7 and Implications for Human Disease. Viruses. 2022 Sep 28:14(10):. doi: 10.3390/v14102138. Epub 2022 Sep 28
[PubMed PMID: 36298698]
[14]
Choi PW, Liu TL, Wong CW, Liu SK, Lum YL, Ming WK. The Dysregulation of MicroRNAs in the Development of Cervical Pre-Cancer-An Update. International journal of molecular sciences. 2022 Jun 27:23(13):. doi: 10.3390/ijms23137126. Epub 2022 Jun 27
[PubMed PMID: 35806128]
[15]
Nowakowski A, Cybulski M, Buda I, Janosz I, Olszak-Wąsik K, Bodzek P, Śliwczyński A, Teter Z, Olejek A, Baranowski W. Cervical Cancer Histology, Staging and Survival before and after Implementation of Organised Cervical Screening Programme in Poland. PloS one. 2016:11(5):e0155849. doi: 10.1371/journal.pone.0155849. Epub 2016 May 19
[PubMed PMID: 27196050]
[16]
Lax S. Histopathology of cervical precursor lesions and cancer. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2011 Sep:20(3):125-33
[PubMed PMID: 22131112]
[18]
Zhang S, Xu H, Zhang L, Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2020 Dec 31:32(6):720-728. doi: 10.21147/j.issn.1000-9604.2020.06.05. Epub
[PubMed PMID: 33446995]
Level 2 (mid-level) evidence
[19]
Hutchcraft ML, Miller RW. Bleeding from Gynecologic Malignancies. Obstetrics and gynecology clinics of North America. 2022 Sep:49(3):607-622. doi: 10.1016/j.ogc.2022.02.022. Epub
[PubMed PMID: 36122988]
[20]
Zhdan VM, Holovanova IA, Vovk OY, Korosh MV. RELATIONSHIP BETWEEN CERVICAL CANCER AND THE LEVEL OF PREVENTIVE ONCOLOGICAL EXAMINATIONS. Wiadomosci lekarskie (Warsaw, Poland : 1960). 2021:74(6):1428-1432
[PubMed PMID: 34159932]
[21]
Burness JV, Schroeder JM, Warren JB. Cervical Colposcopy: Indications and Risk Assessment. American family physician. 2020 Jul 1:102(1):39-48
[PubMed PMID: 32603071]
[22]
Mirpour S, Mhlanga JC, Logeswaran P, Russo G, Mercier G, Subramaniam RM. The role of PET/CT in the management of cervical cancer. AJR. American journal of roentgenology. 2013 Aug:201(2):W192-205. doi: 10.2214/AJR.12.9830. Epub
[PubMed PMID: 23883234]
[23]
Senol T, Polat M, Ozkaya E, Karateke A. Comparison of Two Step LEEP and Cold Conisation For Cervical Intraepithelial Lesions to Decrease Positive Surgical Margins. Asian Pacific journal of cancer prevention : APJCP. 2016:17(7):3317-20
[PubMed PMID: 27509969]
[24]
Shachner TR, Van Meter SE. Metastatic melanoma of the uterine cervix diagnosed on cervical Pap smear: Case report and literature review. Diagnostic cytopathology. 2018 Dec:46(12):1045-1049. doi: 10.1002/dc.24058. Epub 2018 Oct 24
[PubMed PMID: 30354020]
Level 3 (low-level) evidence
[25]
Larsson G, Gullberg B, Grundsell H. A comparison of complications of laser and cold knife conization. Obstetrics and gynecology. 1983 Aug:62(2):213-7
[PubMed PMID: 6408545]
[26]
Brun JL, Youbi A, Hocké C. [Complications, sequellae and outcome of cervical conizations: evaluation of three surgical technics]. Journal de gynecologie, obstetrique et biologie de la reproduction. 2002 Oct:31(6):558-64
[PubMed PMID: 12407327]
[27]
Smith ES, Moon AS, O'Hanlon R, Leitao MM Jr, Sonoda Y, Abu-Rustum NR, Mueller JJ. Radical Trachelectomy for the Treatment of Early-Stage Cervical Cancer: A Systematic Review. Obstetrics and gynecology. 2020 Sep:136(3):533-542. doi: 10.1097/AOG.0000000000003952. Epub
[PubMed PMID: 32769648]
Level 1 (high-level) evidence
[28]
Cibula D, Abu-Rustum NR, Benedetti-Panici P, Köhler C, Raspagliesi F, Querleu D, Morrow CP. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecologic oncology. 2011 Aug:122(2):264-8. doi: 10.1016/j.ygyno.2011.04.029. Epub 2011 May 17
[PubMed PMID: 21592548]
[29]
Querleu D, Morrow CP. Classification of radical hysterectomy. The Lancet. Oncology. 2008 Mar:9(3):297-303. doi: 10.1016/S1470-2045(08)70074-3. Epub
[PubMed PMID: 18308255]
[30]
Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, Isla D, Tamura M, Zhu T, Robledo KP, Gebski V, Asher R, Behan V, Nicklin JL, Coleman RL, Obermair A. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. The New England journal of medicine. 2018 Nov 15:379(20):1895-1904. doi: 10.1056/NEJMoa1806395. Epub 2018 Oct 31
[PubMed PMID: 30380365]
[31]
Li RZ, Sun LF, Li R, Wang HJ. Survival after minimally invasive radical hysterectomy without using uterine manipulator for early-stage cervical cancer: A systematic review and meta-analysis. BJOG : an international journal of obstetrics and gynaecology. 2023 Jan:130(2):176-183. doi: 10.1111/1471-0528.17339. Epub 2022 Nov 13
[PubMed PMID: 36331008]
Level 1 (high-level) evidence
[32]
Bogani G, Di Donato V, Scambia G, Raspagliesi F, Chiantera V, Sozzi G, Golia D'Augè T, Muzii L, Benedetti Panici P, D'Oria O, Vizza E, Giannini A, On Behalf Of The Investigators Of The Italian Gynecological Cancer Study Group. Radical Hysterectomy for Early Stage Cervical Cancer. International journal of environmental research and public health. 2022 Sep 15:19(18):. doi: 10.3390/ijerph191811641. Epub 2022 Sep 15
[PubMed PMID: 36141917]
[33]
Touhami O, Plante M. Minimally Invasive Surgery for Cervical Cancer in Light of the LACC Trial: What Have We Learned? Current oncology (Toronto, Ont.). 2022 Feb 14:29(2):1093-1106. doi: 10.3390/curroncol29020093. Epub 2022 Feb 14
[PubMed PMID: 35200592]
[34]
Lécuru F, Mathevet P, Querleu D, Leblanc E, Morice P, Daraï E, Marret H, Magaud L, Gillaizeau F, Chatellier G, Dargent D. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: results of the SENTICOL study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1:29(13):1686-91. doi: 10.1200/JCO.2010.32.0432. Epub 2011 Mar 28
[PubMed PMID: 21444878]
[35]
Mathevet P, Lécuru F, Uzan C, Boutitie F, Magaud L, Guyon F, Querleu D, Fourchotte V, Baron M, Bats AS, Senticol 2 group. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). European journal of cancer (Oxford, England : 1990). 2021 May:148():307-315. doi: 10.1016/j.ejca.2021.02.009. Epub 2021 Mar 24
[PubMed PMID: 33773275]
Level 1 (high-level) evidence
[36]
Baboudjian M, Gondran-Tellier B, Michel F, Abdallah R, Rouy M, Gaillet S, Sichez PC, Boissier R, Bladou F, Lechevallier E, Karsenty G. Miami Pouch: A Simple Technique for Efficient Continent Cutaneous Urinary Diversion. Urology. 2021 Jun:152():178-183. doi: 10.1016/j.urology.2021.02.004. Epub 2021 Feb 10
[PubMed PMID: 33581233]
[37]
von Knobloch R, Seybold M, Fischer HP, Kibele M, Samad WA. Modification of the Indiana Pouch Ileo-Caecal Cutaneous Continent Urinary Diversion: Tubular Ileal Afferent Limb for Ureteral Anastomosis Has Low Stricture Rate and Allows Ileal Ureter Replacement. Urologia internationalis. 2022:106(2):180-185. doi: 10.1159/000518561. Epub 2021 Sep 23
[PubMed PMID: 34569528]
[38]
Matsuzaki S, Klar M, Chang EJ, Matsuzaki S, Maeda M, Zhang RH, Roman LD, Matsuo K. Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy. Journal of clinical medicine. 2021 Oct 19:10(20):. doi: 10.3390/jcm10204787. Epub 2021 Oct 19
[PubMed PMID: 34682910]
[39]
Bizzarri N, Chiantera V, Ercoli A, Fagotti A, Tortorella L, Conte C, Cappuccio S, Di Donna MC, Gallotta V, Scambia G, Vizzielli G. Minimally Invasive Pelvic Exenteration for Gynecologic Malignancies: A Multi-Institutional Case Series and Review of the Literature. Journal of minimally invasive gynecology. 2019 Nov-Dec:26(7):1316-1326. doi: 10.1016/j.jmig.2018.12.019. Epub 2019 Jan 4
[PubMed PMID: 30611973]
Level 2 (mid-level) evidence
[40]
Westin SN, Rallapalli V, Fellman B, Urbauer DL, Pal N, Frumovitz MM, Ramondetta LM, Bodurka DC, Ramirez PT, Soliman PT. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecologic oncology. 2014 Sep:134(3):546-51. doi: 10.1016/j.ygyno.2014.06.034. Epub 2014 Jul 9
[PubMed PMID: 25014540]
[41]
Höckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. The Lancet. Oncology. 2006 Oct:7(10):837-47
[PubMed PMID: 17012046]
Level 2 (mid-level) evidence
[42]
Magrina JF, Stanhope CR, Weaver AL. Pelvic exenterations: supralevator, infralevator, and with vulvectomy. Gynecologic oncology. 1997 Jan:64(1):130-5
[PubMed PMID: 8995561]
[43]
Bourke J, Wong K, Srinivasjois R, Pereira G, Shepherd CCJ, White SW, Stanley F, Leonard H. Predicting Long-Term Survival Without Major Disability for Infants Born Preterm. The Journal of pediatrics. 2019 Dec:215():90-97.e1. doi: 10.1016/j.jpeds.2019.07.056. Epub 2019 Sep 4
[PubMed PMID: 31493909]
[44]
Zanotti-Fregonara P, Laforest R, Wallis JW. Fetal Radiation Dose from 18F-FDG in Pregnant Patients Imaged with PET, PET/CT, and PET/MR. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015 Aug:56(8):1218-22. doi: 10.2967/jnumed.115.157032. Epub 2015 Jun 18
[PubMed PMID: 26089550]
[45]
Alouini S, Rida K, Mathevet P. Cervical cancer complicating pregnancy: implications of laparoscopic lymphadenectomy. Gynecologic oncology. 2008 Mar:108(3):472-7. doi: 10.1016/j.ygyno.2007.12.006. Epub 2008 Jan 16
[PubMed PMID: 18201752]
[46]
Bernardini F, Ferrandina G, Ricci C, Fagotti A, Fanfani F, Cavaliere AF, Gui B, Scambia G, De Vincenzo R. Neoadjuvant Chemotherapy in Pregnant Patients with Cervical Cancer: A Monocentric Retrospective Study. Current oncology (Toronto, Ont.). 2022 Aug 14:29(8):5702-5714. doi: 10.3390/curroncol29080450. Epub 2022 Aug 14
[PubMed PMID: 36005188]
Level 2 (mid-level) evidence
[47]
Amant F, Berveiller P, Boere IA, Cardonick E, Fruscio R, Fumagalli M, Halaska MJ, Hasenburg A, Johansson ALV, Lambertini M, Lok CAR, Maggen C, Morice P, Peccatori F, Poortmans P, Van Calsteren K, Vandenbroucke T, van Gerwen M, van den Heuvel-Eibrink M, Zagouri F, Zapardiel I. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Annals of oncology : official journal of the European Society for Medical Oncology. 2019 Oct 1:30(10):1601-1612. doi: 10.1093/annonc/mdz228. Epub
[PubMed PMID: 31435648]
Level 3 (low-level) evidence
[48]
Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, Favini G, Ferri L, Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet (London, England). 1997 Aug 23:350(9077):535-40
[PubMed PMID: 9284774]
Level 1 (high-level) evidence
[49]
Landoni F, Colombo A, Milani R, Placa F, Zanagnolo V, Mangioni C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update. Journal of gynecologic oncology. 2017 May:28(3):e34. doi: 10.3802/jgo.2017.28.e34. Epub 2017 Feb 24
[PubMed PMID: 28382797]
Level 1 (high-level) evidence
[50]
van Kol K, Ebisch R, Piek J, Beugeling M, Vergeldt T, Bekkers R. Adjuvant Hysterectomy for Cervical Cancer Patients Treated with Chemoradiation Therapy: A Systematic Review on the Pathology-Proven Residual Disease Rate. Cancers. 2021 Dec 8:13(24):. doi: 10.3390/cancers13246190. Epub 2021 Dec 8
[PubMed PMID: 34944810]
Level 1 (high-level) evidence
[51]
Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. The New England journal of medicine. 1999 Apr 15:340(15):1137-43
[PubMed PMID: 10202164]
[52]
Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecologic oncology. 1999 May:73(2):177-83
[PubMed PMID: 10329031]
Level 1 (high-level) evidence
[53]
Levinson K, Beavis AL, Purdy C, Rositch AF, Viswanathan A, Wolfson AH, Kelly MG, Tewari KS, McNally L, Guntupalli SR, Ragab O, Lee YC, Miller DS, Huh WK, Wilkinson KJ, Spirtos NM, Van Le L, Casablanca Y, Holman LL, Waggoner SE, Fader AN. Beyond Sedlis-A novel histology-specific nomogram for predicting cervical cancer recurrence risk: An NRG/GOG ancillary analysis. Gynecologic oncology. 2021 Sep:162(3):532-538. doi: 10.1016/j.ygyno.2021.06.017. Epub 2021 Jul 1
[PubMed PMID: 34217544]
[54]
Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000 Apr:18(8):1606-13
[PubMed PMID: 10764420]
[55]
Huang H, Feng YL, Wan T, Zhang YN, Cao XP, Huang YW, Xiong Y, Huang X, Zheng M, Li YF, Li JD, Chen GD, Li H, Chen YL, Ma LG, Yang HY, Li L, Yao SZ, Ye WJ, Tu H, Huang QD, Liang LZ, Liu FY, Liu Q, Liu JH. Effectiveness of Sequential Chemoradiation vs Concurrent Chemoradiation or Radiation Alone in Adjuvant Treatment After Hysterectomy for Cervical Cancer: The STARS Phase 3 Randomized Clinical Trial. JAMA oncology. 2021 Mar 1:7(3):361-369. doi: 10.1001/jamaoncol.2020.7168. Epub
[PubMed PMID: 33443541]
Level 1 (high-level) evidence
[56]
Mell LK, Sirák I, Wei L, Tarnawski R, Mahantshetty U, Yashar CM, McHale MT, Xu R, Honerkamp-Smith G, Carmona R, Wright M, Williamson CW, Kasaová L, Li N, Kry S, Michalski J, Bosch W, Straube W, Schwarz J, Lowenstein J, Jiang SB, Saenz CC, Plaxe S, Einck J, Khorprasert C, Koonings P, Harrison T, Shi M, Mundt AJ, INTERTECC Study Group. Bone Marrow-sparing Intensity Modulated Radiation Therapy With Concurrent Cisplatin For Stage IB-IVA Cervical Cancer: An International Multicenter Phase II Clinical Trial (INTERTECC-2). International journal of radiation oncology, biology, physics. 2017 Mar 1:97(3):536-545. doi: 10.1016/j.ijrobp.2016.11.027. Epub 2016 Nov 23
[PubMed PMID: 28126303]
Level 1 (high-level) evidence
[57]
Yeung AR, Pugh SL, Klopp AH, Gil KM, Wenzel L, Westin SN, Gaffney DK, Small W Jr, Thompson S, Doncals DE, Cantuaria GHC, Yaremko BP, Chang A, Kundapur V, Mohan DS, Haas ML, Kim YB, Ferguson CL, Deshmukh S, Bruner DW, Kachnic LA. Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 May 20:38(15):1685-1692. doi: 10.1200/JCO.19.02381. Epub 2020 Feb 19
[PubMed PMID: 32073955]
[58]
Gonzalez VJ, Hullett CR, Burt L, Rassiah-Szegedi P, Sarkar V, Tward JD, Hazard LJ, Huang YJ, Salter BJ, Gaffney DK. Impact of prone versus supine positioning on small bowel dose with pelvic intensity modulated radiation therapy. Advances in radiation oncology. 2017 Apr-Jun:2(2):235-243. doi: 10.1016/j.adro.2017.01.005. Epub 2017 Jan 24
[PubMed PMID: 28740937]
Level 3 (low-level) evidence
[59]
Small W Jr, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, Jhingran A, Portelance L, Schefter T, Iyer R, Varia M, Winter K, Mundt AJ. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. International journal of radiation oncology, biology, physics. 2008 Jun 1:71(2):428-34
[PubMed PMID: 18037584]
Level 3 (low-level) evidence
[60]
Ríos I, Vásquez I, Cuervo E, Garzón Ó, Burbano J. Problems and solutions in IGRT for cervical cancer. Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2018 Nov-Dec:23(6):517-527. doi: 10.1016/j.rpor.2018.05.002. Epub 2018 May 26
[PubMed PMID: 30534015]
[61]
Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, Miften M. Radiation dose-volume effects in the stomach and small bowel. International journal of radiation oncology, biology, physics. 2010 Mar 1:76(3 Suppl):S101-7. doi: 10.1016/j.ijrobp.2009.05.071. Epub
[PubMed PMID: 20171503]
[62]
Viswanathan AN, Thomadsen B, American Brachytherapy Society Cervical Cancer Recommendations Committee, American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012 Jan-Feb:11(1):33-46. doi: 10.1016/j.brachy.2011.07.003. Epub
[PubMed PMID: 22265436]
Level 3 (low-level) evidence
[63]
Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, Jones E, Kirisits C, Thomadsen B, Erickson B, American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012 Jan-Feb:11(1):47-52. doi: 10.1016/j.brachy.2011.07.002. Epub
[PubMed PMID: 22265437]
Level 3 (low-level) evidence
[64]
Charra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, Castelain B, Thomas L, Peiffert D. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: results of the French STIC prospective study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 Jun:103(3):305-13. doi: 10.1016/j.radonc.2012.04.007. Epub 2012 May 25
[PubMed PMID: 22633469]
[65]
Pötter R, Tanderup K, Schmid MP, Jürgenliemk-Schulz I, Haie-Meder C, Fokdal LU, Sturdza AE, Hoskin P, Mahantshetty U, Segedin B, Bruheim K, Huang F, Rai B, Cooper R, van der Steen-Banasik E, Van Limbergen E, Pieters BR, Tan LT, Nout RA, De Leeuw AAC, Ristl R, Petric P, Nesvacil N, Kirchheiner K, Kirisits C, Lindegaard JC, EMBRACE Collaborative Group. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. The Lancet. Oncology. 2021 Apr:22(4):538-547. doi: 10.1016/S1470-2045(20)30753-1. Epub
[PubMed PMID: 33794207]
[66]
Dimopoulos JC, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, Pedersen EM, van Limbergen E, Haie-Meder C, Pötter R. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 Apr:103(1):113-22. doi: 10.1016/j.radonc.2011.12.024. Epub 2012 Jan 30
[PubMed PMID: 22296748]
Level 2 (mid-level) evidence
[67]
Banerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. International journal of women's health. 2014:6():555-64. doi: 10.2147/IJWH.S46247. Epub 2014 May 28
[PubMed PMID: 24920937]
[68]
Liu R, Wang X, Tian JH, Yang K, Wang J, Jiang L, Hao XY. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. The Cochrane database of systematic reviews. 2014 Oct 9:2014(10):CD007563. doi: 10.1002/14651858.CD007563.pub3. Epub 2014 Oct 9
[PubMed PMID: 25300170]
Level 1 (high-level) evidence
[70]
Mazeron R, Fokdal LU, Kirchheiner K, Georg P, Jastaniyah N, Šegedin B, Mahantshetty U, Hoskin P, Jürgenliemk-Schulz I, Kirisits C, Lindegaard JC, Dörr W, Haie-Meder C, Tanderup K, Pötter R, EMBRACE collaborative group. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2016 Sep:120(3):412-419. doi: 10.1016/j.radonc.2016.06.006. Epub 2016 Jul 7
[PubMed PMID: 27396811]
[71]
Lucidarme D, Marteau P, Foucault M, Vautrin B, Filoche B. Efficacy and tolerance of mesalazine suppositories vs. hydrocortisone foam in proctitis. Alimentary pharmacology & therapeutics. 1997 Apr:11(2):335-40
[PubMed PMID: 9146772]
[72]
Rigaud J, Hetet JF, Bouchot O. [Management of radiation cystitis]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2004 Sep:14(4):568-72
[PubMed PMID: 15776916]
[73]
Pereira D, Ferreira C, Catarino R, Correia T, Cardoso A, Reis F, Cerqueira M, Prisco R, Camacho O. Hyperbaric oxygen for radiation-induced cystitis: A long-term follow-up. Actas urologicas espanolas. 2020 Oct:44(8):561-567. doi: 10.1016/j.acuro.2020.03.010. Epub 2020 Jul 28
[PubMed PMID: 32736899]
[74]
Boice JD Jr, Day NE, Andersen A, Brinton LA, Brown R, Choi NW, Clarke EA, Coleman MP, Curtis RE, Flannery JT. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. Journal of the National Cancer Institute. 1985 May:74(5):955-75
[PubMed PMID: 3858584]
[75]
Wright JD, St Clair CM, Deutsch I, Burke WM, Gorrochurn P, Sun X, Herzog TJ. Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer. 2010 May 15:116(10):2486-92. doi: 10.1002/cncr.25067. Epub
[PubMed PMID: 20209618]
[76]
Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. International journal of radiation oncology, biology, physics. 2005 Jul 1:62(3):738-44
[PubMed PMID: 15936554]
[77]
Small W Jr, Kim YS, Joyce C, Surucu M, Leshyk M, Harkenrider MM, Potkul RK, Liotta M, Winder A, Altoos B. Uterine perforation during brachytherapy for cervical cancer: Complications, outcomes, and best practices for forward treatment planning and management. Brachytherapy. 2021 May-Jun:20(3):557-564. doi: 10.1016/j.brachy.2021.02.001. Epub 2021 Mar 17
[PubMed PMID: 33741275]
[78]
van Dyk S, Schneider M, Kondalsamy-Chennakesavan S, Bernshaw D, Narayan K. Ultrasound use in gynecologic brachytherapy: Time to focus the beam. Brachytherapy. 2015 May-Jun:14(3):390-400. doi: 10.1016/j.brachy.2014.12.001. Epub 2015 Jan 22
[PubMed PMID: 25620161]
[79]
Rowlands S, Oloto E, Horwell DH. Intrauterine devices and risk of uterine perforation: current perspectives. Open access journal of contraception. 2016:7():19-32. doi: 10.2147/OAJC.S85546. Epub 2016 Mar 16
[PubMed PMID: 29386934]
Level 3 (low-level) evidence
[80]
Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. The New England journal of medicine. 1999 Apr 15:340(15):1144-53
[PubMed PMID: 10202165]
[81]
Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, Pattaranutaporn P, Hameed S, Blair JM, Barraclough H, Orlando M. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1:29(13):1678-85. doi: 10.1200/JCO.2009.25.9663. Epub 2011 Mar 28
[PubMed PMID: 21444871]
Level 1 (high-level) evidence
[82]
Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, Miller DS, Olt G, King S, Boggess JF, Rocereto TF. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Aug 1:22(15):3113-9
[PubMed PMID: 15284262]
[83]
Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Oct 1:27(28):4649-55. doi: 10.1200/JCO.2009.21.8909. Epub 2009 Aug 31
[PubMed PMID: 19720909]
[84]
Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, DiSaia PJ, Copeland LJ, Creasman WT, Stehman FB, Brady MF, Burger RA, Thigpen JT, Birrer MJ, Waggoner SE, Moore DH, Look KY, Koh WJ, Monk BJ. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet (London, England). 2017 Oct 7:390(10103):1654-1663. doi: 10.1016/S0140-6736(17)31607-0. Epub 2017 Jul 27
[PubMed PMID: 28756902]
Level 1 (high-level) evidence
[85]
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Jan 1:38(1):1-10. doi: 10.1200/JCO.19.02105. Epub 2019 Nov 4
[PubMed PMID: 31682550]
[86]
Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E, Yañez E, Gümüş M, Olivera Hurtado de Mendoza M, Samouëlian V, Castonguay V, Arkhipov A, Toker S, Li K, Keefe SM, Monk BJ, KEYNOTE-826 Investigators. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. The New England journal of medicine. 2021 Nov 11:385(20):1856-1867. doi: 10.1056/NEJMoa2112435. Epub 2021 Sep 18
[PubMed PMID: 34534429]
[87]
Nishio S, Yonemori K, Usami T, Minobe S, Yunokawa M, Iwata T, Okamoto A, Aoki Y, Itamochi H, Takekuma M, Harano K, Yamamoto K, Maruko T, Ugai H, Tekin C, Colombo N, Fujiwara K, Hasegawa K, Ushijima K. Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer science. 2022 Nov:113(11):3877-3887. doi: 10.1111/cas.15479. Epub 2022 Sep 15
[PubMed PMID: 35792064]
[88]
Kitagawa R, Katsumata N, Shibata T, Kamura T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M, Satoh T, Yoshikawa H. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Jul 1:33(19):2129-35. doi: 10.1200/JCO.2014.58.4391. Epub 2015 Mar 2
[PubMed PMID: 25732161]
Level 1 (high-level) evidence
[89]
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2021 Oct:155 Suppl 1(Suppl 1):28-44. doi: 10.1002/ijgo.13865. Epub
[PubMed PMID: 34669203]
[90]
Saleh M, Virarkar M, Javadi S, Elsherif SB, de Castro Faria S, Bhosale P. Cervical Cancer: 2018 Revised International Federation of Gynecology and Obstetrics Staging System and the Role of Imaging. AJR. American journal of roentgenology. 2020 May:214(5):1182-1195. doi: 10.2214/AJR.19.21819. Epub 2020 Mar 17
[PubMed PMID: 32182097]
[91]
Salib MY, Russell JHB, Stewart VR, Sudderuddin SA, Barwick TD, Rockall AG, Bharwani N. 2018 FIGO Staging Classification for Cervical Cancer: Added Benefits of Imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2020 Oct:40(6):1807-1822. doi: 10.1148/rg.2020200013. Epub 2020 Sep 18
[PubMed PMID: 32946322]
[92]
Castellano T, Ding K, Moore KN, Landrum LM. Simple Hysterectomy for Cervical Cancer: Risk Factors for Failed Screening and Deviation From Screening Guidelines. Journal of lower genital tract disease. 2019 Apr:23(2):124-128. doi: 10.1097/LGT.0000000000000463. Epub
[PubMed PMID: 30817687]
[93]
Chen HH, Meng WY, Li RZ, Wang QY, Wang YW, Pan HD, Yan PY, Wu QB, Liu L, Yao XJ, Kang M, Leung EL. Potential prognostic factors in progression-free survival for patients with cervical cancer. BMC cancer. 2021 May 10:21(1):531. doi: 10.1186/s12885-021-08243-3. Epub 2021 May 10
[PubMed PMID: 33971846]
[94]
Rauh-Hain JA, Clemmer JT, Bradford LS, Clark RM, Growdon WB, Goodman A, Boruta DM 2nd, Schorge JO, del Carmen MG. Racial disparities in cervical cancer survival over time. Cancer. 2013 Oct 15:119(20):3644-52. doi: 10.1002/cncr.28261. Epub 2013 Jul 31
[PubMed PMID: 23913530]
[95]
Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019 Jan-Feb:18(1):29-37. doi: 10.1016/j.brachy.2018.08.016. Epub 2018 Oct 22
[PubMed PMID: 30361045]
[96]
Pergialiotis V, Bellos I, Thomakos N, Haidopoulos D, Perrea DN, Kontzoglou K, Daskalakis G, Rodolakis A. Survival outcomes of patients with cervical cancer and accompanying hydronephrosis: A systematic review of the literature. Oncology reviews. 2019 Jan 14:13(1):387. doi: 10.4081/oncol.2019.387. Epub 2019 Jan 15
[PubMed PMID: 30746036]
Level 1 (high-level) evidence
[97]
Mendu S, Boukhechba M, Gordon JR, Datta D, Molina E, Arroyo G, Proctor SK, Wells KJ, Barnes LE. Design of a Culturally-Informed Virtual Human for Educating Hispanic Women about Cervical Cancer. International Conference on Pervasive Computing Technologies for Healthcare : [proceedings]. International Conference on Pervasive Computing Technologies for Healthcare. 2018 May:2018():360-366. doi: 10.1145/3240925.3240968. Epub
[PubMed PMID: 30555731]
[98]
Nasser S, Berek J, Ullrich A, Giordano L, Sehouli J. A report on the Marrakech International Women's Cancer Days: dialogs and implications. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2019 Feb:29(2):417-421. doi: 10.1136/ijgc-2018-000059. Epub 2018 Dec 21
[PubMed PMID: 30718317]
Level 2 (mid-level) evidence
[99]
Cunningham-Erves J, Forbes L, Ivankova N, Mayo-Gamble T, Kelly-Taylor K, Deakings J. Black mother's intention to vaccinate daughters against HPV: A mixed methods approach to identify opportunities for targeted communication. Gynecologic oncology. 2018 Jun:149(3):506-512. doi: 10.1016/j.ygyno.2018.03.047. Epub 2018 Mar 24
[PubMed PMID: 29588103]
[100]
Mabelele MM, Materu J, Ng'ida FD, Mahande MJ. Knowledge towards cervical cancer prevention and screening practices among women who attended reproductive and child health clinic at Magu district hospital, Lake Zone Tanzania: a cross-sectional study. BMC cancer. 2018 May 16:18(1):565. doi: 10.1186/s12885-018-4490-7. Epub 2018 May 16
[PubMed PMID: 29769124]
Level 2 (mid-level) evidence
[101]
Lai D, Bodson J, Davis FA, Lee D, Tavake-Pasi F, Napia E, Villalta J, Mukundente V, Mooney R, Coulter H, Stark LA, Sanchez-Birkhead AC, Kepka D. Diverse Families' Experiences with HPV Vaccine Information Sources: A Community-Based Participatory Approach. Journal of community health. 2017 Apr:42(2):400-412. doi: 10.1007/s10900-016-0269-4. Epub
[PubMed PMID: 27734247]
[102]
Spinner C, Ding L, Bernstein DI, Brown DR, Franco EL, Covert C, Kahn JA. Human Papillomavirus Vaccine Effectiveness and Herd Protection in Young Women. Pediatrics. 2019 Feb:143(2):. doi: 10.1542/peds.2018-1902. Epub 2019 Jan 22
[PubMed PMID: 30670582]
[103]
Bayu H, Berhe Y, Mulat A, Alemu A. Cervical Cancer Screening Service Uptake and Associated Factors among Age Eligible Women in Mekelle Zone, Northern Ethiopia, 2015: A Community Based Study Using Health Belief Model. PloS one. 2016:11(3):e0149908. doi: 10.1371/journal.pone.0149908. Epub 2016 Mar 10
[PubMed PMID: 26963098]
[104]
Kung TH, Gordon JR, Abdullahi A, Barve A, Chaudhari V, Kosambiya JK, Kumar A, Gamit S, Wells KJ. "My husband says this: If you are alive, you can be someone…": Facilitators and barriers to cervical cancer screening among women living with HIV in India. Cancer causes & control : CCC. 2019 Apr:30(4):365-374. doi: 10.1007/s10552-019-01145-7. Epub 2019 Feb 26
[PubMed PMID: 30809741]
[105]
McPherson GS, Fairbairn-Dunlop P, Payne D. Overcoming Barriers to Cervical Screening Among Pacific Women: A Narrative Review. Health equity. 2019:3(1):22-29. doi: 10.1089/heq.2018.0076. Epub 2019 Feb 14
[PubMed PMID: 30783634]
Level 3 (low-level) evidence