Introduction
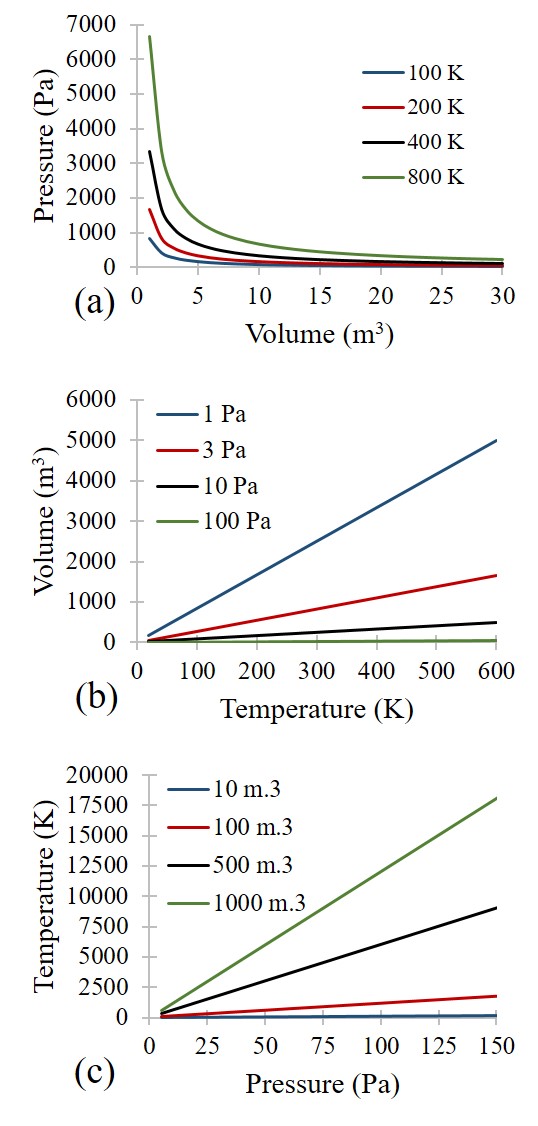
The Ideal Gas Law is a simple equation demonstrating the relationship between temperature, pressure, and volume for gases. These specific relationships stem from Charles’s Law, Boyle’s Law, and Gay-Lussac’s Law. Charles’s Law identifies the direct proportionality between volume and temperature at constant pressure, Boyle’s Law identifies the inverse proportionality of pressure and volume at a constant temperature, and Gay-Lussac’s Law identifies the direct proportionality of pressure and temperature at constant volume. Combined, these form the Ideal Gas Law equation: PV = NRT. P is the pressure, V is the volume, N is the number of moles of gas, R is the universal gas constant, and T is the absolute temperature.
The universal gas constant R is a number that satisfies the proportionalities of the pressure-volume-temperature relationship. R has different values and units that depend on the user’s pressure, volume, moles, and temperature specifications. Various values for R are on online databases, or the user can use dimensional analysis to convert the observed units of pressure, volume, moles, and temperature to match a known R-value. As long as the units are consistent, either approach is acceptable. The temperature value in the Ideal Gas Law must be in absolute units (Rankine [degrees R] or Kelvin [K]) to prevent the right-hand side from being zero, which violates the pressure-volume-temperature relationship. The conversion to absolute temperature units is a simple addition to either the Fahrenheit (F) or the Celsius (C) temperature: Degrees R = F + 459.67 and K = C + 273.15.
For a gas to be “ideal” there are four governing assumptions:
- The gas particles have negligible volume.
- The gas particles are equally sized and do not have intermolecular forces (attraction or repulsion) with other gas particles.
- The gas particles move randomly in agreement with Newton’s Laws of Motion.
- The gas particles have perfect elastic collisions with no energy loss.
In reality, there are no ideal gases. Any gas particle possesses a volume within the system (a minute amount, but present nonetheless), which violates the first assumption. Additionally, gas particles can be of different sizes; for example, hydrogen gas is significantly smaller than xenon gas. Gases in a system do have intermolecular forces with neighboring gas particles, especially at low temperatures where the particles are not moving quickly and interact with each other. Even though gas particles can move randomly, they do not have perfect elastic collisions due to the conservation of energy and momentum within the system.[1][2][3]
While ideal gases are strictly a theoretical conception, real gases can behave ideally under certain conditions. Systems with either very low pressures or high temperatures enable real gases to be estimated as “ideal.” The low pressure of a system allows the gas particles to experience less intermolecular forces with other gas particles. Similarly, high-temperature systems allow for the gas particles to move quickly within the system and exhibit less intermolecular forces with each other. Therefore, for calculation purposes, real gases can be considered “ideal” in either low pressure or high-temperature systems.
The Ideal Gas Law also holds true for a system containing multiple ideal gases; this is known as an ideal gas mixture. With multiple ideal gases in a system, these particles are still assumed not to have any intermolecular interactions with one another. An ideal gas mixture partitions the total pressure of the system into the partial pressure contributions of each of the different gas particles. This allows for the previous ideal gas equation to be re-written: Pi·V = ni·R·T. In this equation, Pi is the partial pressure of species i and ni are the moles of species i. At low pressure or high-temperature conditions, gas mixtures can be considered ideal gas mixtures for ease of calculation.
When systems are not at low pressures or high temperatures, the gas particles are able to interact with one another; these interactions greatly inhibit the Ideal Gas Law’s accuracy. There are, however, other models, such as the Van der Waals Equation of State, that account for the volume of the gas particles and the intermolecular interactions. The discussion beyond the Ideal Gas Law is outside the scope of this article.