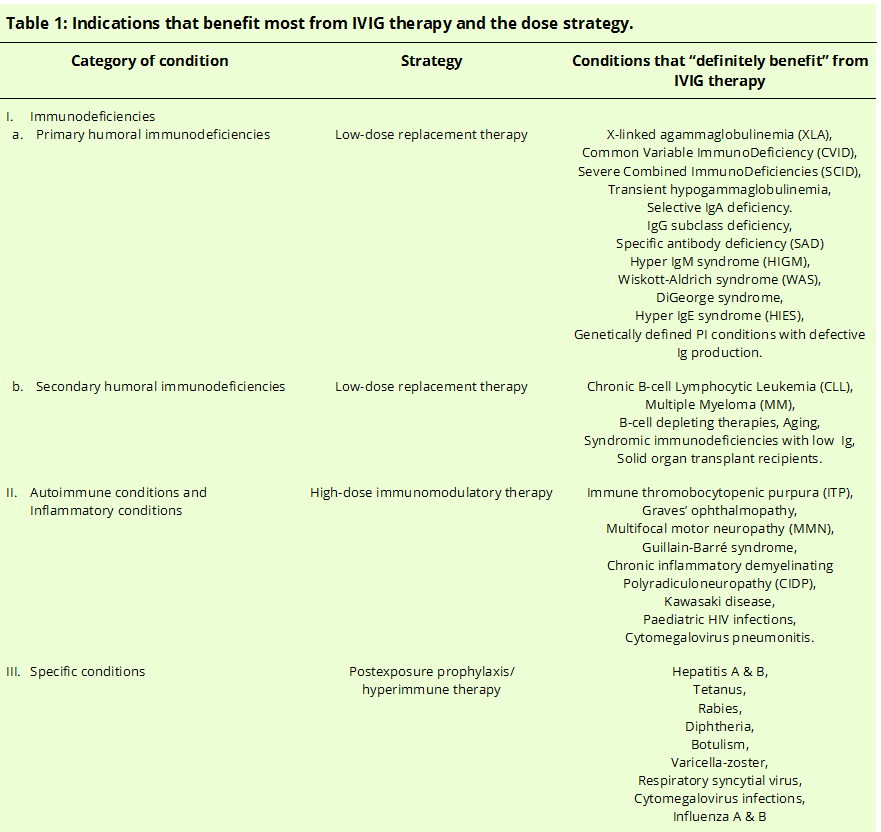
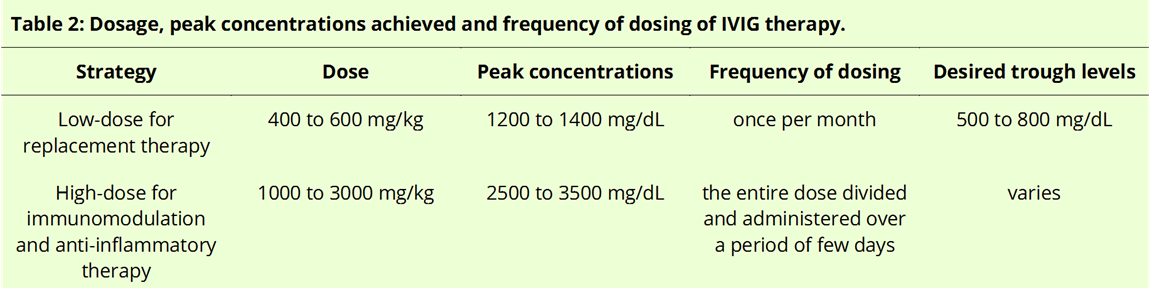
[3]
Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. Journal of immunology (Baltimore, Md. : 1950). 2015 Jan 1:194(1):13-20. doi: 10.4049/jimmunol.1400844. Epub
[PubMed PMID: 25527792]
[4]
Loh RK, Vale S, McLean-Tooke A. Quantitative serum immunoglobulin tests. Australian family physician. 2013 Apr:42(4):195-8
[PubMed PMID: 23550242]
[5]
Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Petersen PH, Johnson AM, Milford-Ward A, Ritchie RF, Svendsen PJ, Whicher J. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies. 1996 Jun:34(6):517-20
[PubMed PMID: 8831057]
Level 3 (low-level) evidence
[6]
Barahona Afonso AF, João CM. The Production Processes and Biological Effects of Intravenous Immunoglobulin. Biomolecules. 2016 Mar 9:6(1):15. doi: 10.3390/biom6010015. Epub 2016 Mar 9
[PubMed PMID: 27005671]
[7]
Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox sanguinis. 2010 Jan:98(1):12-28. doi: 10.1111/j.1423-0410.2009.01226.x. Epub 2009 Jul 29
[PubMed PMID: 19660029]
Level 2 (mid-level) evidence
[8]
Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, Nelson R, Secord E, Jordan SC, Stiehm ER, Vo AA, Ballow M. Update on the use of immunoglobulin in human disease: A review of evidence. The Journal of allergy and clinical immunology. 2017 Mar:139(3S):S1-S46. doi: 10.1016/j.jaci.2016.09.023. Epub 2016 Dec 29
[PubMed PMID: 28041678]
[9]
Modell V, Knaus M, Modell F, Roifman C, Orange J, Notarangelo LD. Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunologic research. 2014 Oct:60(1):132-44. doi: 10.1007/s12026-014-8498-z. Epub
[PubMed PMID: 24668296]
Level 3 (low-level) evidence
[10]
Reda SM, El-Ghoneimy DH, Afifi HM. Clinical predictors of primary immunodeficiency diseases in children. Allergy, asthma & immunology research. 2013 Mar:5(2):88-95. doi: 10.4168/aair.2013.5.2.88. Epub 2012 Nov 2
[PubMed PMID: 23450209]
[12]
El-Sayed ZA, Abramova I, Aldave JC, Al-Herz W, Bezrodnik L, Boukari R, Bousfiha AA, Cancrini C, Condino-Neto A, Dbaibo G, Derfalvi B, Dogu F, Edgar JDM, Eley B, El-Owaidy RH, Espinosa-Padilla SE, Galal N, Haerynck F, Hanna-Wakim R, Hossny E, Ikinciogullari A, Kamal E, Kanegane H, Kechout N, Lau YL, Morio T, Moschese V, Neves JF, Ouederni M, Paganelli R, Paris K, Pignata C, Plebani A, Qamar FN, Qureshi S, Radhakrishnan N, Rezaei N, Rosario N, Routes J, Sanchez B, Sediva A, Seppanen MR, Serrano EG, Shcherbina A, Singh S, Siniah S, Spadaro G, Tang M, Vinet AM, Volokha A, Sullivan KE. X-linked agammaglobulinemia (XLA):Phenotype, diagnosis, and therapeutic challenges around the world. The World Allergy Organization journal. 2019:12(3):100018. doi: 10.1016/j.waojou.2019.100018. Epub 2019 Mar 22
[PubMed PMID: 30937141]
[13]
De Ranieri D, Fenny NS. Intravenous Immunoglobulin in the Treatment of Primary Immunodeficiency Diseases. Pediatric annals. 2017 Jan 1:46(1):e8-e12. doi: 10.3928/19382359-20161213-03. Epub
[PubMed PMID: 28079912]
[14]
Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. The Journal of allergy and clinical immunology. 2010 Feb:125(2 Suppl 2):S195-203. doi: 10.1016/j.jaci.2009.08.040. Epub 2009 Dec 29
[PubMed PMID: 20042227]
[15]
Cunningham-Rundles C. How I treat common variable immune deficiency. Blood. 2010 Jul 8:116(1):7-15. doi: 10.1182/blood-2010-01-254417. Epub 2010 Mar 23
[PubMed PMID: 20332369]
[16]
Tjon AS, van Gent R, Geijtenbeek TB, Kwekkeboom J. Differences in Anti-Inflammatory Actions of Intravenous Immunoglobulin between Mice and Men: More than Meets the Eye. Frontiers in immunology. 2015:6():197. doi: 10.3389/fimmu.2015.00197. Epub 2015 Apr 28
[PubMed PMID: 25972869]
[17]
Cooper N, Ghanima W. Immune Thrombocytopenia. The New England journal of medicine. 2019 Sep 5:381(10):945-955. doi: 10.1056/NEJMcp1810479. Epub
[PubMed PMID: 31483965]
[18]
Hemming VG. Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases. Clinical and diagnostic laboratory immunology. 2001 Sep:8(5):859-63
[PubMed PMID: 11527792]
[20]
Bournazos S, Ravetch JV. Diversification of IgG effector functions. International immunology. 2017 Jul 1:29(7):303-310. doi: 10.1093/intimm/dxx025. Epub
[PubMed PMID: 28472280]
[21]
Bournazos S, Ravetch JV. Fcγ receptor pathways during active and passive immunization. Immunological reviews. 2015 Nov:268(1):88-103. doi: 10.1111/imr.12343. Epub
[PubMed PMID: 26497515]
[22]
Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nature reviews. Immunology. 2013 Mar:13(3):176-89. doi: 10.1038/nri3401. Epub 2013 Feb 15
[PubMed PMID: 23411799]
[23]
Basta M. Ambivalent effect of immunoglobulins on the complement system: activation versus inhibition. Molecular immunology. 2008 Oct:45(16):4073-9. doi: 10.1016/j.molimm.2008.07.012. Epub 2008 Aug 15
[PubMed PMID: 18706699]
[24]
Chaigne B, Mouthon L. Mechanisms of action of intravenous immunoglobulin. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2017 Feb:56(1):45-49. doi: 10.1016/j.transci.2016.12.017. Epub 2016 Dec 30
[PubMed PMID: 28161150]
[25]
Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clinical and experimental immunology. 2011 Jun:164 Suppl 2(Suppl 2):2-5. doi: 10.1111/j.1365-2249.2011.04387.x. Epub
[PubMed PMID: 21466545]
[26]
Paquin-Proulx D, Santos BA, Carvalho KI, Toledo-Barros M, Barreto de Oliveira AK, Kokron CM, Kalil J, Moll M, Kallas EG, Sandberg JK. IVIg immune reconstitution treatment alleviates the state of persistent immune activation and suppressed CD4 T cell counts in CVID. PloS one. 2013:8(10):e75199. doi: 10.1371/journal.pone.0075199. Epub 2013 Oct 9
[PubMed PMID: 24130688]
[27]
Bayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Sibéril S, Dimitrov JD, Lacroix-Desmazes S, Berdah M, Crabol Y, Oksenhendler E, Lévy Y, Mouthon L, Sautès-Fridman C, Hermine O, Kaveri SV. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. Journal of autoimmunity. 2011 Feb:36(1):9-15. doi: 10.1016/j.jaut.2010.09.006. Epub 2010 Dec 9
[PubMed PMID: 20970960]
[28]
Quinti I, Mitrevski M. Modulatory Effects of Antibody Replacement Therapy to Innate and Adaptive Immune Cells. Frontiers in immunology. 2017:8():697. doi: 10.3389/fimmu.2017.00697. Epub 2017 Jun 16
[PubMed PMID: 28670314]
[29]
Ben Mkaddem S, Benhamou M, Monteiro RC. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Frontiers in immunology. 2019:10():811. doi: 10.3389/fimmu.2019.00811. Epub 2019 Apr 12
[PubMed PMID: 31057544]
Level 3 (low-level) evidence
[31]
Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. The Journal of experimental medicine. 2007 Jan 22:204(1):11-5
[PubMed PMID: 17227911]
[32]
Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, Komorowski L, Luo J, Cabral-Marques O, Hammers CM, Lindstrom JM, Lamprecht P, Fischer A, Riemekasten G, Tersteeg C, Sondermann P, Rapoport B, Wandinger KP, Probst C, El Beidaq A, Schmidt E, Verkman A, Manz RA, Nimmerjahn F. Mechanisms of Autoantibody-Induced Pathology. Frontiers in immunology. 2017:8():603. doi: 10.3389/fimmu.2017.00603. Epub 2017 May 31
[PubMed PMID: 28620373]
[33]
Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annual review of immunology. 2008:26():513-33. doi: 10.1146/annurev.immunol.26.021607.090232. Epub
[PubMed PMID: 18370923]
[34]
Sultan Y, Kazatchkine MD, Maisonneuve P, Nydegger UE. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet (London, England). 1984 Oct 6:2(8406):765-8
[PubMed PMID: 6148519]
[35]
Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Molecular immunology. 2009 Sep:46(14):2753-66. doi: 10.1016/j.molimm.2009.04.027. Epub 2009 May 28
[PubMed PMID: 19477527]
[36]
Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, Szebeni J, Alving CR, Carroll MC, Berkower I, Stojilkovic SS, Metcalfe DD. F(ab)'2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nature medicine. 2003 Apr:9(4):431-8
[PubMed PMID: 12612546]
[37]
Basta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. The Journal of clinical investigation. 1994 Nov:94(5):1729-35
[PubMed PMID: 7962520]
[38]
Nagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the Role of Fc-Gamma Receptors: Classic Mechanisms of Action after all? Frontiers in immunology. 2014:5():674. doi: 10.3389/fimmu.2014.00674. Epub 2015 Jan 21
[PubMed PMID: 25653650]
[39]
Li X, Kimberly RP. Targeting the Fc receptor in autoimmune disease. Expert opinion on therapeutic targets. 2014 Mar:18(3):335-50. doi: 10.1517/14728222.2014.877891. Epub
[PubMed PMID: 24521454]
Level 3 (low-level) evidence
[40]
Bussel JB. Fc receptor blockade and immune thrombocytopenic purpura. Seminars in hematology. 2000 Jul:37(3):261-6
[PubMed PMID: 10942220]
[41]
Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proceedings of the National Academy of Sciences of the United States of America. 1996 May 28:93(11):5512-6
[PubMed PMID: 8643606]
[42]
Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011 Jun 19:475(7354):110-3. doi: 10.1038/nature10134. Epub 2011 Jun 19
[PubMed PMID: 21685887]
[43]
Kozicky LK, Zhao ZY, Menzies SC, Fidanza M, Reid GS, Wilhelmsen K, Hellman J, Hotte N, Madsen KL, Sly LM. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. Journal of leukocyte biology. 2015 Dec:98(6):983-94. doi: 10.1189/jlb.3VMA0315-078R. Epub 2015 Jul 27
[PubMed PMID: 26216934]
[44]
Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, Nutt SL, Hu X, Ivashkiv LB. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007 Jan:26(1):67-78
[PubMed PMID: 17239631]
[45]
Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, Chevailler A, Mouthon L, Weill B, Bruneval P, Kazatchkine MD, Kaveri SV. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003 Jan 15:101(2):758-65
[PubMed PMID: 12393386]
[46]
Bayry J, Lacroix-Desmazes S, Delignat S, Mouthon L, Weill B, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-alpha present in serum from patients with systemic lupus erythematosus. Arthritis and rheumatism. 2003 Dec:48(12):3497-502
[PubMed PMID: 14674000]
[47]
Casulli S, Topçu S, Fattoum L, von Gunten S, Simon HU, Teillaud JL, Bayry J, Kaveri SV, Elbim C. A differential concentration-dependent effect of IVIg on neutrophil functions: relevance for anti-microbial and anti-inflammatory mechanisms. PloS one. 2011:6(10):e26469. doi: 10.1371/journal.pone.0026469. Epub 2011 Oct 31
[PubMed PMID: 22065996]
[48]
von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, Simon HU. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. The Journal of allergy and clinical immunology. 2007 Apr:119(4):1005-11
[PubMed PMID: 17337295]
[49]
Prasad NK, Papoff G, Zeuner A, Bonnin E, Kazatchkine MD, Ruberti G, Kaveri SV. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. Journal of immunology (Baltimore, Md. : 1950). 1998 Oct 1:161(7):3781-90
[PubMed PMID: 9759905]
[50]
Maddur MS, Sharma M, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibitory effect of IVIG on IL-17 production by Th17 cells is independent of anti-IL-17 antibodies in the immunoglobulin preparations. Journal of clinical immunology. 2013 Jan:33 Suppl 1():S62-6. doi: 10.1007/s10875-012-9752-6. Epub 2012 Aug 7
[PubMed PMID: 22864643]
[51]
Massoud AH, Guay J, Shalaby KH, Bjur E, Ablona A, Chan D, Nouhi Y, McCusker CT, Mourad MW, Piccirillo CA, Mazer BD. Intravenous immunoglobulin attenuates airway inflammation through induction of forkhead box protein 3-positive regulatory T cells. The Journal of allergy and clinical immunology. 2012 Jun:129(6):1656-65.e3. doi: 10.1016/j.jaci.2012.02.050. Epub 2012 May 5
[PubMed PMID: 22564681]
[52]
Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, Delignat S, Elluru S, Bayry J, Lacroix-Desmazes S, Cohen JL, Salomon BL, Kazatchkine MD, Kaveri SV, Misra N. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008 Jan 15:111(2):715-22
[PubMed PMID: 17932250]
[53]
Hori A, Fujimura T, Kawamoto S. Anti-inflammatory intravenous immunoglobulin (IVIg) suppresses homeostatic proliferation of B cells. Cytotechnology. 2018 Jun:70(3):921-927. doi: 10.1007/s10616-017-0176-2. Epub 2018 Apr 2
[PubMed PMID: 29611058]
[54]
Le Pottier L, Sapir T, Bendaoud B, Youinou P, Shoenfeld Y, Pers JO. Intravenous immunoglobulin and cytokines: focus on tumor necrosis factor family members BAFF and APRIL. Annals of the New York Academy of Sciences. 2007 Sep:1110():426-32
[PubMed PMID: 17911457]
[55]
Xu C, Poirier B, Duong Van Huyen JP, Lucchiari N, Michel O, Chevalier J, Kaveri S. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. The American journal of pathology. 1998 Oct:153(4):1257-66
[PubMed PMID: 9777957]
[56]
Cho YT, Chu CY. Treatments for Severe Cutaneous Adverse Reactions. Journal of immunology research. 2017:2017():1503709. doi: 10.1155/2017/1503709. Epub 2017 Dec 27
[PubMed PMID: 29445753]
[57]
Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H, Garofalo K, Meccariello R, Meador JW 3rd, Rutitzky L, Schultes BC, Ling L, Avery W, Nimmerjahn F, Manning AM, Kaundinya GV, Bosques CJ. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proceedings of the National Academy of Sciences of the United States of America. 2015 Mar 17:112(11):E1297-306. doi: 10.1073/pnas.1422481112. Epub 2015 Mar 2
[PubMed PMID: 25733881]
[58]
Hsu JL, Safdar N. Polyclonal immunoglobulins and hyperimmune globulins in prevention and management of infectious diseases. Infectious disease clinics of North America. 2011 Dec:25(4):773-88. doi: 10.1016/j.idc.2011.07.005. Epub
[PubMed PMID: 22054755]
[59]
Toubi E, Etzioni A. Intravenous immunoglobulin in immunodeficiency states: state of the art. Clinical reviews in allergy & immunology. 2005 Dec:29(3):167-72
[PubMed PMID: 16391391]
[60]
Notarangelo LD. Primary immunodeficiencies. The Journal of allergy and clinical immunology. 2010 Feb:125(2 Suppl 2):S182-94. doi: 10.1016/j.jaci.2009.07.053. Epub 2009 Dec 29
[PubMed PMID: 20042228]
[61]
Rojavin MA, Hubsch A, Lawo JP. Quantitative Evidence of Wear-Off Effect at the End of the Intravenous IgG (IVIG) Dosing Cycle in Primary Immunodeficiency. Journal of clinical immunology. 2016 Apr:36(3):210-9. doi: 10.1007/s10875-016-0243-z. Epub 2016 Feb 24
[PubMed PMID: 26910102]
[62]
Erlendsson K, Swartz T, Dwyer JM. Successful reversal of echovirus encephalitis in X-linked hypogammaglobulinemia by intraventricular administration of immunoglobulin. The New England journal of medicine. 1985 Feb 7:312(6):351-3
[PubMed PMID: 4038544]
[63]
Patwa HS. Dosing and individualized treatment - patient-centric treatment: changing practice guidelines. Clinical and experimental immunology. 2014 Dec:178 Suppl 1(Suppl 1):36-8. doi: 10.1111/cei.12503. Epub
[PubMed PMID: 25546754]
Level 1 (high-level) evidence
[64]
Zandman-Goddard G, Krauthammer A, Levy Y, Langevitz P, Shoenfeld Y. Long-term therapy with intravenous immunoglobulin is beneficial in patients with autoimmune diseases. Clinical reviews in allergy & immunology. 2012 Apr:42(2):247-55. doi: 10.1007/s12016-011-8278-7. Epub
[PubMed PMID: 21732045]
[65]
Khan AM, Mydra H, Nevarez A. Clinical Practice Updates in the Management Of Immune Thrombocytopenia. P & T : a peer-reviewed journal for formulary management. 2017 Dec:42(12):756-763
[PubMed PMID: 29234214]
[66]
Kareva L, Mironska K, Stavric K, Hasani A. Adverse Reactions to Intravenous Immunoglobulins - Our Experience. Open access Macedonian journal of medical sciences. 2018 Dec 20:6(12):2359-2362. doi: 10.3889/oamjms.2018.513. Epub 2018 Dec 17
[PubMed PMID: 30607191]
[67]
Koleba T, Ensom MH. Pharmacokinetics of intravenous immunoglobulin: a systematic review. Pharmacotherapy. 2006 Jun:26(6):813-27
[PubMed PMID: 16716135]
Level 1 (high-level) evidence
[68]
Bonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunology and allergy clinics of North America. 2008 Nov:28(4):803-19, ix. doi: 10.1016/j.iac.2008.06.006. Epub
[PubMed PMID: 18940575]
[69]
Wasserman RL, Church JA, Peter HH, Sleasman JW, Melamed I, Stein MR, Bichler J, IgPro10 in PID Study group. Pharmacokinetics of a new 10% intravenous immunoglobulin in patients receiving replacement therapy for primary immunodeficiency. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2009 Jun 28:37(3-4):272-8. doi: 10.1016/j.ejps.2009.02.014. Epub 2009 Mar 6
[PubMed PMID: 19491015]
[70]
Shehata N,Palda V,Bowen T,Haddad E,Issekutz TB,Mazer B,Schellenberg R,Warrington R,Easton D,Anderson D,Hume H, The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfusion medicine reviews. 2010 Jan;
[PubMed PMID: 19962579]
Level 1 (high-level) evidence
[71]
Bonagura VR. Using intravenous immunoglobulin (IVIG) to treat patients with primary immune deficiency disease. Journal of clinical immunology. 2013 Jan:33 Suppl 2():S90-4. doi: 10.1007/s10875-012-9838-1. Epub 2012 Dec 28
[PubMed PMID: 23271459]
[72]
Gardulf A, Björvell H, Andersen V, Björkander J, Ericson D, Frøland SS, Gustafson R, Hammarström L, Nyström T, Søeberg B. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients' experiences of subcutaneous self-infusions and home therapy. Journal of advanced nursing. 1995 May:21(5):917-27
[PubMed PMID: 7541407]
[73]
Kobayashi RH, Gupta S, Melamed I, Mandujano JF, Kobayashi AL, Ritchie B, Geng B, Atkinson TP, Rehman S, Turpel-Kantor E, Litzman J. Clinical Efficacy, Safety and Tolerability of a New Subcutaneous Immunoglobulin 16.5% (Octanorm [Cutaquig®]) in the Treatment of Patients With Primary Immunodeficiencies. Frontiers in immunology. 2019:10():40. doi: 10.3389/fimmu.2019.00040. Epub 2019 Feb 4
[PubMed PMID: 30778345]
[74]
Guo Y, Tian X, Wang X, Xiao Z. Adverse Effects of Immunoglobulin Therapy. Frontiers in immunology. 2018:9():1299. doi: 10.3389/fimmu.2018.01299. Epub 2018 Jun 8
[PubMed PMID: 29951056]
[75]
Palabrica FR, Kwong SL, Padua FR. Adverse events of intravenous immunoglobulin infusions: a ten-year retrospective study. Asia Pacific allergy. 2013 Oct:3(4):249-56. doi: 10.5415/apallergy.2013.3.4.249. Epub 2013 Oct 31
[PubMed PMID: 24260730]
Level 2 (mid-level) evidence
[76]
Siegel J. The product: All intravenous immunoglobulins are not equivalent. Pharmacotherapy. 2005 Nov:25(11 Pt 2):78S-84S
[PubMed PMID: 16229678]
[77]
Stein MR. The new generation of liquid intravenous immunoglobulin formulations in patient care: a comparison of intravenous immunoglobulins. Postgraduate medicine. 2010 Sep:122(5):176-84. doi: 10.3810/pgm.2010.09.2214. Epub
[PubMed PMID: 20861601]
[79]
Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. The New England journal of medicine. 1986 Feb 27:314(9):560-4
[PubMed PMID: 3945295]
[80]
Rachid R, Castells M, Cunningham-Rundles C, Bonilla FA. Association of anti-IgA antibodies with adverse reactions to γ-globulin infusion. The Journal of allergy and clinical immunology. 2011 Jul:128(1):228-230.e1. doi: 10.1016/j.jaci.2011.01.061. Epub 2011 Mar 11
[PubMed PMID: 21397310]
[81]
Björkander J, Hammarström L, Smith CI, Buckley RH, Cunningham-Rundles C, Hanson LA. Immunoglobulin prophylaxis in patients with antibody deficiency syndromes and anti-IgA antibodies. Journal of clinical immunology. 1987 Jan:7(1):8-15
[PubMed PMID: 3494039]
[82]
Tacke CE, Smits GP, van der Klis FR, Kuipers IM, Zaaijer HL, Kuijpers TW. Reduced serologic response to mumps, measles, and rubella vaccination in patients treated with intravenous immunoglobulin for Kawasaki disease. The Journal of allergy and clinical immunology. 2013 Jun:131(6):1701-3. doi: 10.1016/j.jaci.2013.01.045. Epub 2013 Mar 14
[PubMed PMID: 23498596]
[83]
Moulis G, Sailler L, Sommet A, Lapeyre-Mestre M, Montastruc JL, French Association of Regional Pharmacovigilance Centers. Exposure to inhibitors of the renin-angiotensin system is a major independent risk factor for acute renal failure induced by sucrose-containing intravenous immunoglobulins: a case-control study. Pharmacoepidemiology and drug safety. 2012 Mar:21(3):314-9. doi: 10.1002/pds.2253. Epub 2011 Sep 28
[PubMed PMID: 21953992]
Level 2 (mid-level) evidence
[84]
Jolles S, Orange JS, Gardulf A, Stein MR, Shapiro R, Borte M, Berger M. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clinical and experimental immunology. 2015 Feb:179(2):146-60. doi: 10.1111/cei.12485. Epub
[PubMed PMID: 25384609]
[85]
Kahwaji J, Barker E, Pepkowitz S, Klapper E, Villicana R, Peng A, Chang R, Jordan SC, Vo AA. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clinical journal of the American Society of Nephrology : CJASN. 2009 Dec:4(12):1993-7. doi: 10.2215/CJN.04540709. Epub 2009 Oct 15
[PubMed PMID: 19833910]
[86]
Dantal J. Intravenous immunoglobulins: in-depth review of excipients and acute kidney injury risk. American journal of nephrology. 2013:38(4):275-84. doi: 10.1159/000354893. Epub 2013 Sep 14
[PubMed PMID: 24051350]
[87]
Binitha MP, Nandakumar G, Thomas D. Suspected cardiac toxicity to intravenous immunoglobulin used for treatment of scleromyxedema. Indian journal of dermatology, venereology and leprology. 2008 May-Jun:74(3):248-50
[PubMed PMID: 18583794]
[88]
Baxley A, Akhtari M. Hematologic toxicities associated with intravenous immunoglobulin therapy. International immunopharmacology. 2011 Nov:11(11):1663-7. doi: 10.1016/j.intimp.2011.07.024. Epub 2011 Aug 16
[PubMed PMID: 21843660]