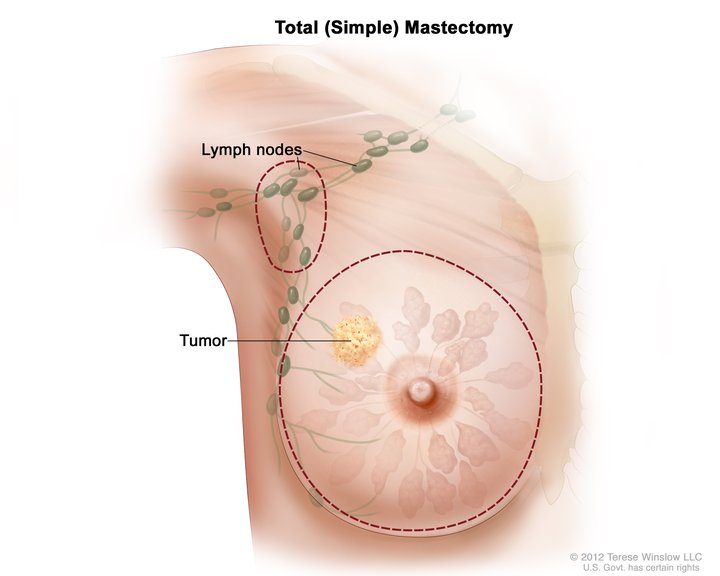
[1]
Mota BS, Bevilacqua JLB, Barrett J, Ricci MD, Munhoz AM, Filassi JR, Baracat EC, Riera R. Skin-sparing mastectomy for the treatment of breast cancer. The Cochrane database of systematic reviews. 2023 Mar 27:3(3):CD010993. doi: 10.1002/14651858.CD010993.pub2. Epub 2023 Mar 27
[PubMed PMID: 36972145]
Level 1 (high-level) evidence
[2]
Sakorafas GH. The origins of radical mastectomy. AORN journal. 2008 Oct:88(4):605-8. doi: 10.1016/j.aorn.2008.06.001. Epub
[PubMed PMID: 18928961]
[3]
Duncan AM, Al Youha S, Joukhadar N, Konder R, Stecco C, Wheelock ME. Anatomy of the Breast Fascial System: A Systematic Review of the Literature. Plastic and reconstructive surgery. 2022 Jan 1:149(1):28-40. doi: 10.1097/PRS.0000000000008671. Epub
[PubMed PMID: 34936599]
Level 1 (high-level) evidence
[4]
Vegas MR, Martina L, Segovia-Gonzalez M, Garcia-Garcia JF, Gonzalez-Gonzalez A, Mendieta-Baro A, Nieto-Gongora C, Benito-Duque P. Vascular anatomy of the breast and its implications in the breast-sharing reconstruction technique. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2023 Jan:76():180-188. doi: 10.1016/j.bjps.2022.10.021. Epub 2022 Oct 17
[PubMed PMID: 36521264]
[5]
Machado P, Liu JB, Needleman L, Lee C, Forsberg F. Anatomy Versus Physiology: Is Breast Lymphatic Drainage to the Internal Thoracic (Internal Mammary) Lymphatic System Clinically Relevant? Journal of breast cancer. 2023 Jun:26(3):286-291. doi: 10.4048/jbc.2023.26.e16. Epub 2023 Mar 31
[PubMed PMID: 37272244]
[6]
Cardoso MJ, de Boniface J, Dodwell D, Kaidar-Person O, Poortmans P, van Maaren MC. Which real indications remain for mastectomy? Lancet regional health. Americas. 2024 May:33():100734. doi: 10.1016/j.lana.2024.100734. Epub 2024 Apr 4
[PubMed PMID: 38590325]
[7]
Ciabattoni A, Gregucci F, De Rose F, Falivene S, Fozza A, Daidone A, Morra A, Smaniotto D, Barbara R, Lozza L, Vidali C, Borghesi S, Palumbo I, Huscher A, Perrucci E, Baldissera A, Tolento G, Rovea P, Franco P, De Santis MC, Grazia AD, Marino L, Meduri B, Cucciarelli F, Aristei C, Bertoni F, Guenzi M, Leonardi MC, Livi L, Nardone L, De Felice F, Rosetto ME, Mazzuoli L, Anselmo P, Arcidiacono F, Barbarino R, Martinetti M, Pasinetti N, Desideri I, Marazzi F, Ivaldi G, Bonzano E, Cavallari M, Cerreta V, Fusco V, Sarno L, Bonanni A, Mangiacotti MG, Prisco A, Buonfrate G, Andrulli D, Fontana A, Bagnoli R, Marinelli L, Reverberi C, Scalabrino G, Corazzi F, Doino D, Di Genesio-Pagliuca M, Lazzari M, Mascioni F, Pace MP, Mazza M, Vitucci P, Spera A, Macchia G, Boccardi M, Evangelista G, Sola B, La Porta MR, Fiorentino A, Levra NG, Ippolito E, Silipigni S, Osti MF, Mignogna M, Alessandro M, Ursini LA, Nuzzo M, Meattini I, D'Ermo G. AIRO Breast Cancer Group Best Clinical Practice 2022 Update. Tumori. 2022 Jul:108(2_suppl):1-144. doi: 10.1177/03008916221088885. Epub
[PubMed PMID: 36112842]
[8]
Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch AM, Lyons J, Mortimer J, Patel SA, Pierce LJ, Rosenberger LH, Rugo HS, Schneider B, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Wei M, Wisinski KB, Young JS, Yeung K, Dwyer MA, Kumar R. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. Journal of the National Comprehensive Cancer Network : JNCCN. 2023 Jun:21(6):594-608. doi: 10.6004/jnccn.2023.0031. Epub
[PubMed PMID: 37308117]
[9]
Miroshnychenko A, Roldan YM, Ibrahim S, Kulatunga-Moruzi C, Dahlin K, Montante S, Couban R, Guyatt G, Brignardello-Petersen R. "Mastectomy for individuals with gender dysphoria below 26 years of age: A systematic review and meta-analysis". Plastic and reconstructive surgery. 2024 Sep 10:():. doi: 10.1097/PRS.0000000000011734. Epub 2024 Sep 10
[PubMed PMID: 39252149]
Level 1 (high-level) evidence
[10]
Kopkash K, Sisco M, Poli E, Seth A, Pesce C. The modern approach to the nipple-sparing mastectomy. Journal of surgical oncology. 2020 Jul:122(1):29-35. doi: 10.1002/jso.25909. Epub 2020 Mar 26
[PubMed PMID: 32219847]
[11]
Valero MG, Muhsen S, Moo TA, Zabor EC, Stempel M, Pusic A, Gemignani ML, Morrow M, Sacchini VS. Increase in Utilization of Nipple-Sparing Mastectomy for Breast Cancer: Indications, Complications, and Oncologic Outcomes. Annals of surgical oncology. 2020 Feb:27(2):344-351. doi: 10.1245/s10434-019-07948-x. Epub 2019 Dec 10
[PubMed PMID: 31823173]
[12]
Plunkett A, Scott TL, Tracy E. Regional anesthesia for breast cancer surgery: which block is best? A review of the current literature. Pain management. 2022 Nov:12(8):943-950. doi: 10.2217/pmt-2022-0048. Epub 2022 Sep 30
[PubMed PMID: 36177958]
[13]
Smith BL, Coopey SB. Nipple-Sparing Mastectomy. Advances in surgery. 2018 Sep:52(1):113-126. doi: 10.1016/j.yasu.2018.03.008. Epub 2018 Jun 20
[PubMed PMID: 30098607]
Level 3 (low-level) evidence
[14]
Jones C, Lancaster R. Evolution of Operative Technique for Mastectomy. The Surgical clinics of North America. 2018 Aug:98(4):835-844. doi: 10.1016/j.suc.2018.04.003. Epub 2018 May 21
[PubMed PMID: 30005777]
[15]
Colwell AS, Christensen JM. Nipple-Sparing Mastectomy and Direct-to-Implant Breast Reconstruction. Plastic and reconstructive surgery. 2017 Nov:140(5S Advances in Breast Reconstruction):44S-50S. doi: 10.1097/PRS.0000000000003949. Epub
[PubMed PMID: 29064921]
Level 3 (low-level) evidence
[16]
Spiekerman van Weezelenburg MA, Daemen JHT, van Kuijk SMJ, van Haaren ERM, Janssen A, Vissers YLJ, Beets GL, van Bastelaar J. Seroma formation after mastectomy: A systematic review and network meta-analysis of different flap fixation techniques. Journal of surgical oncology. 2024 May:129(6):1015-1024. doi: 10.1002/jso.27589. Epub 2024 Jan 21
[PubMed PMID: 38247263]
Level 1 (high-level) evidence
[17]
Tamminen A, Koskivuo I. Preoperative antibiotic prophylaxis in mastectomy: A retrospective comparative analysis of 1413 patients with breast cancer. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2022 Sep:111(3):56-64. doi: 10.1177/14574969221116940. Epub 2022 Aug 24
[PubMed PMID: 36000713]
Level 2 (mid-level) evidence
[18]
Olsen MA, Nickel KB, Fox IK, Margenthaler JA, Ball KE, Mines D, Wallace AE, Fraser VJ. Incidence of Surgical Site Infection Following Mastectomy With and Without Immediate Reconstruction Using Private Insurer Claims Data. Infection control and hospital epidemiology. 2015 Aug:36(8):907-14. doi: 10.1017/ice.2015.108. Epub 2015 Jun 3
[PubMed PMID: 26036877]
[19]
Pagliara D, Schiavone L, Garganese G, Bove S, Montella RA, Costantini M, Rinaldi PM, Bottosso S, Grieco F, Rubino C, Salgarello M, Ribuffo D. Predicting Mastectomy Skin Flap Necrosis: A Systematic Review of Preoperative and Intraoperative Assessment Techniques. Clinical breast cancer. 2023 Apr:23(3):249-254. doi: 10.1016/j.clbc.2022.12.021. Epub 2023 Jan 4
[PubMed PMID: 36725477]
Level 1 (high-level) evidence
[20]
Narusawa E, Sadeghi S, Tane K, Alkhaifi M, Kikawa Y. Updates on the preventions and management of post-mastectomy pain syndrome beyond medical treatment: a comprehensive narrative review. Annals of palliative medicine. 2024 Sep:13(5):1258-1264. doi: 10.21037/apm-24-73. Epub 2024 Aug 16
[PubMed PMID: 39168643]
Level 3 (low-level) evidence