Definition/Introduction
The follicular phase of the female menstrual cycle involves the maturation of ovarian follicles, preparing them for release during ovulation. During the same period, changes occur in the endometrium, leading to the follicular phase being referred to as the proliferative phase.
Issues of Concern
Follicular Phase
The menstrual cycle typically ranges from 21 to 35 days, with an average length of 28 days. Oligomenorrhea refers to infrequent periods with menstrual cycles longer than 35 days, while polymenorrhea describes frequent periods with cycles shorter than 21 days. Notably, the duration of the follicular phase can vary depending on the overall length of the cycle, whereas the luteal phase is usually stable and lasts 14 days. In a 28-day cycle, the follicular phase extends from the first day of menstruation (day 0) to the start of ovulation (day 14).
When the previous menstrual cycle completes and the corpus luteum breaks down, the levels of estrogen, progesterone, and inhibin A decrease. This chain of events triggers positive feedback to the hypothalamus and anterior pituitary, leading to a pulsatile release of gonadotropin-releasing hormone (GnRH) and follicle-stimulating hormone (FSH) into circulation. The increase in FSH stimulates the granulosa cells of the ovaries, prompting the recruitment of several follicles from each ovary. These follicles mature, but only one Graafian follicle will undergo ovulation during that cycle. The rise in FSH also stimulates the secretion of inhibin B by the granulosa cells. Inhibin B subsequently suppresses FSH secretion toward the end of the follicular phase. Inhibin B levels peak during the luteinizing hormone (LH) surge before ovulation and then decline rapidly. Please see StatPearls' companion resource, "Physiology, Menstrual Cycle," for more information.[1]
The level of FSH can vary based on the age of a female. As women age, ovarian function declines, leading to reduced inhibin production in the preceding luteal phase. Lower inhibin levels result in a higher release of FSH compared to younger females. Elevated FSH levels enhance the recruitment of ovarian follicles, potentially increasing the occurrence of more than 1 ovulation per cycle. As follicles are recruited at an increased rate, the overall duration of the follicular phase shortens, and the follicle released for ovulation may be less mature. Due to these age-related changes in the early follicular phase, physicians can evaluate suspected infertility by measuring serum FSH and estradiol levels around day 3 of the cycle. Additionally, ovarian reserve can be assessed by monitoring serum levels of anti-Müllerian hormone (AMH), which is produced by granulosa cells and plays a crucial role in folliculogenesis. AMH levels can be measured at any point during the menstrual cycle.[2][3][4]
The mid-follicular phase begins with a rise in estradiol and inhibin B levels produced by the ovarian follicles in response to increased FSH. This rise results in negative feedback, leading to decreased FSH levels. During this phase, the selection of the follicle destined for ovulation occurs, and this follicle is termed the dominant follicle. Various theories explain how the dominant follicle is determined. One theory suggests that the follicle with the highest number of FSH receptors promotes its own growth and ovulates, while other follicles are suppressed and undergo atresia. Another theory posits that the AMH plays a role in selecting the dominant follicle.[5][6][7][8]
In response to elevated FSH levels during the early follicular phase, granulosa cells proliferate, leading to an increase in FSH receptors in these cells. The higher FSH levels enable granulosa cells to produce estradiol, which in turn stimulates the production of LH receptors in the granulosa cells. With LH receptors currently present, granulosa cells also produce small amounts of progesterone and 17-hydroxyprogesterone. The progesterone released by granulosa cells regulates their proliferation and ultimately slows follicular growth.[9]
As the follicular phase comes to an end, estradiol levels rise rapidly, causing the negative feedback loop to switch to positive feedback. While the exact reason for this switch is unclear, it is believed that kisspeptin neurons may have a role. The positive feedback from estradiol stimulates the hypothalamus and anterior pituitary, triggering a surge in LH, which signals the end of the follicular phase and the onset of ovulation.[10]
Proliferative Phase
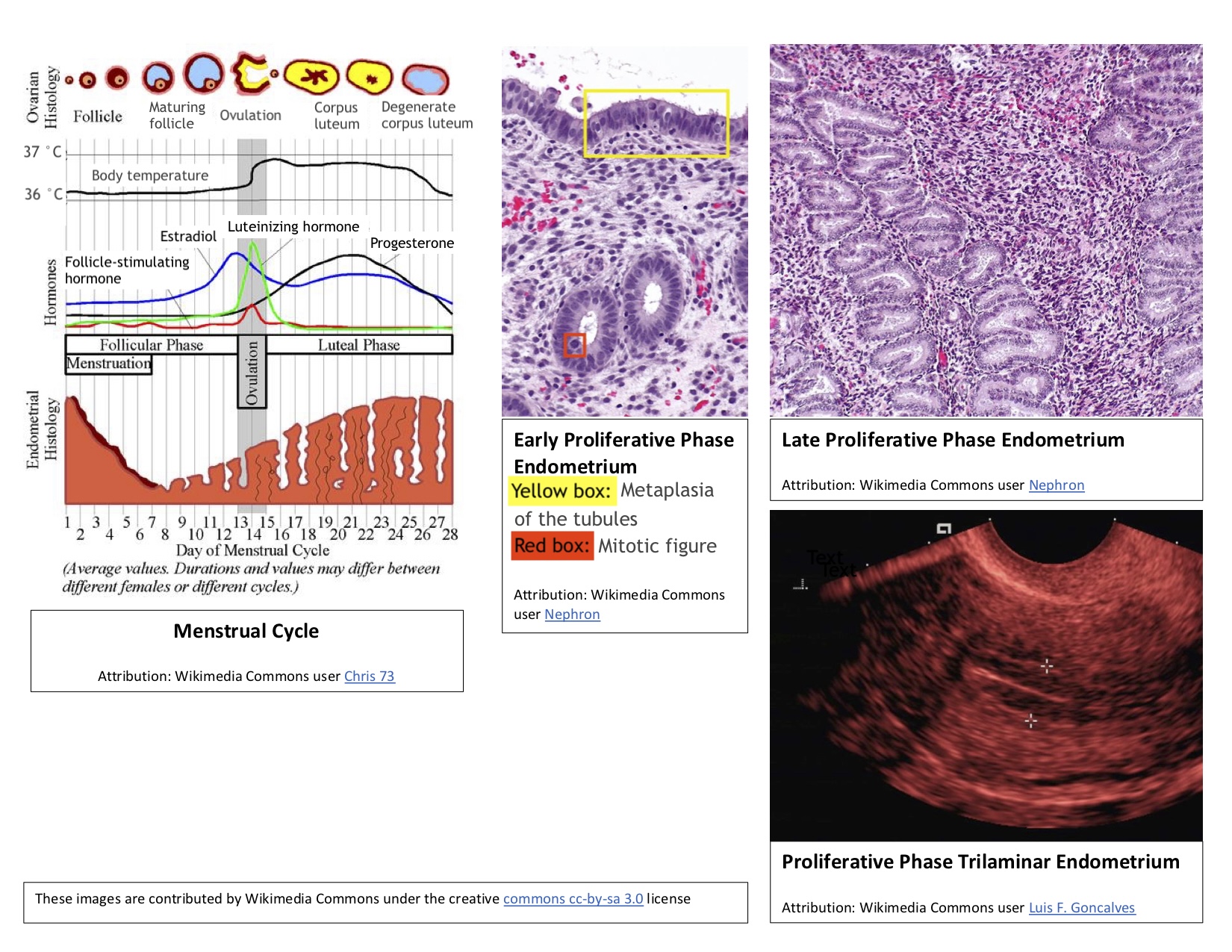
In addition to ovarian follicle maturation, the endometrium undergoes significant changes during the first 14 days of the cycle, which is why this period is referred to as the proliferative phase. Increasing estradiol levels strongly influence these endometrial changes, which occur before ovulation. The proliferative phase can be further divided into early, mid-, and late stages (see Image. Proliferative Phase Endometrium During the Menstrual Cycle).
Early proliferative phase: This phase begins shortly after menstruation, typically around days 4 to 7. During this time, the regenerating endometrium forms a thin, linear, echogenic layer. The glands are short, straight, and narrow, with microvilli and cilia developing on the epithelial cells. Some inactive glands, still recovering from the previous menstrual cycle, may appear cuboidal and ragged. During the early proliferative phase, the densely packed stroma exhibits some mitotic activity, and its cells appear spindle-shaped. The nuclei of these cells enlarge and are surrounded by minimal cytoplasm.
Mid-proliferative phase: The endometrium then progresses to the mid-proliferative phase, typically around days 8 to 10 of the cycle. During this phase, the glands become more elongated and curved and are lined with columnar epithelium.
Late proliferative phase: This phase occurs from approximately day 11 to day 14 of the cycle. The glands become coiled and closely packed during this time and undergo active mitosis and nuclear pseudostratification. The stratum functional layer (also known as the inner lining) of the endometrium reaches its maximum thickness, ranging from 0.5 to 5 mm, and develops a trilaminar appearance. The trilaminar endometrium consists of a thin, echogenic inner line and an echogenic outer basal layer, with a darker rim forming the middle layer. The spiral arteries elongate to ensure adequate blood flow to the endometrium, accommodating the increased endometrial thickness.[1][11]
During the proliferative phase, the cervix undergoes changes in response to the increasing estradiol levels. Cervical crypts produce a thin, watery, mucoid discharge that reduces vaginal acidity. On ultrasonography, the cervical canal appears more dilated and distended to accommodate the increased cervical discharge. Overall, these endometrial and cervical changes work together to create a more welcoming environment for sperm to enter.[12]
Clinical Significance
The median age of menarche in the United States is 12.5 years, with the average female experiencing around 450 menstrual cycles afterward. Clinicians must understand all phases of the menstrual cycle to effectively educate younger patients on its normal range and the bodily changes they may experience at different stages. Early differentiation between normal and abnormal menstrual cycles allows clinicians to diagnose and manage unusual patterns and irregularities in subsequent cycles, such as dysmenorrhea, amenorrhea, menorrhagia, infertility, and other conditions.[13]