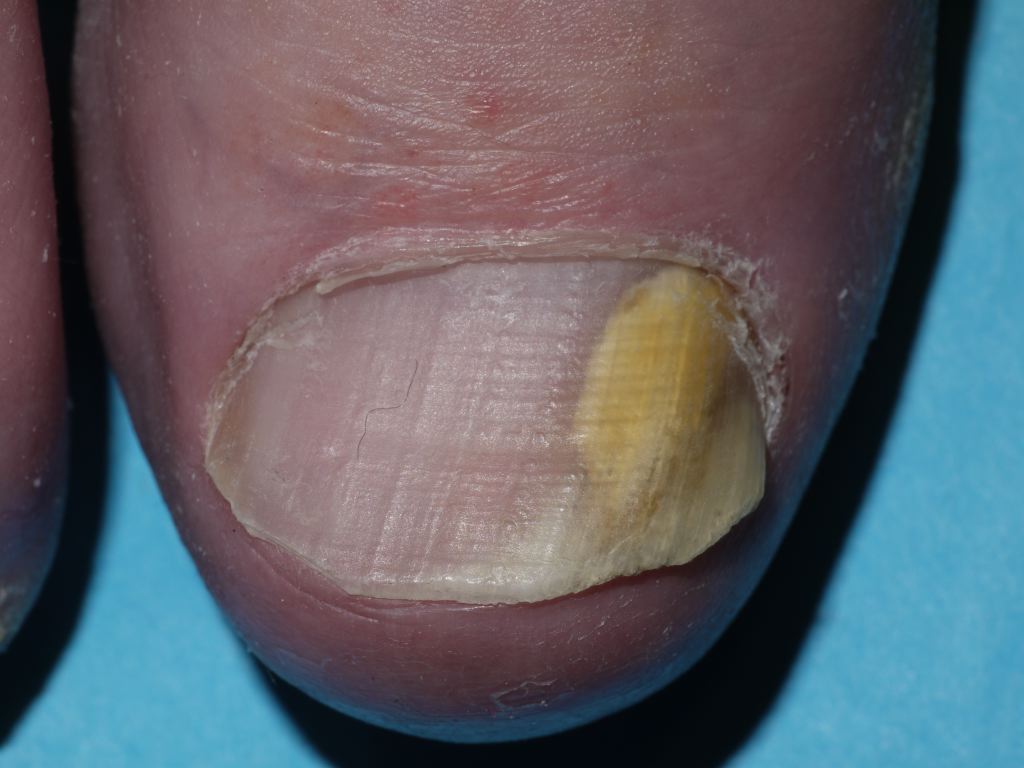
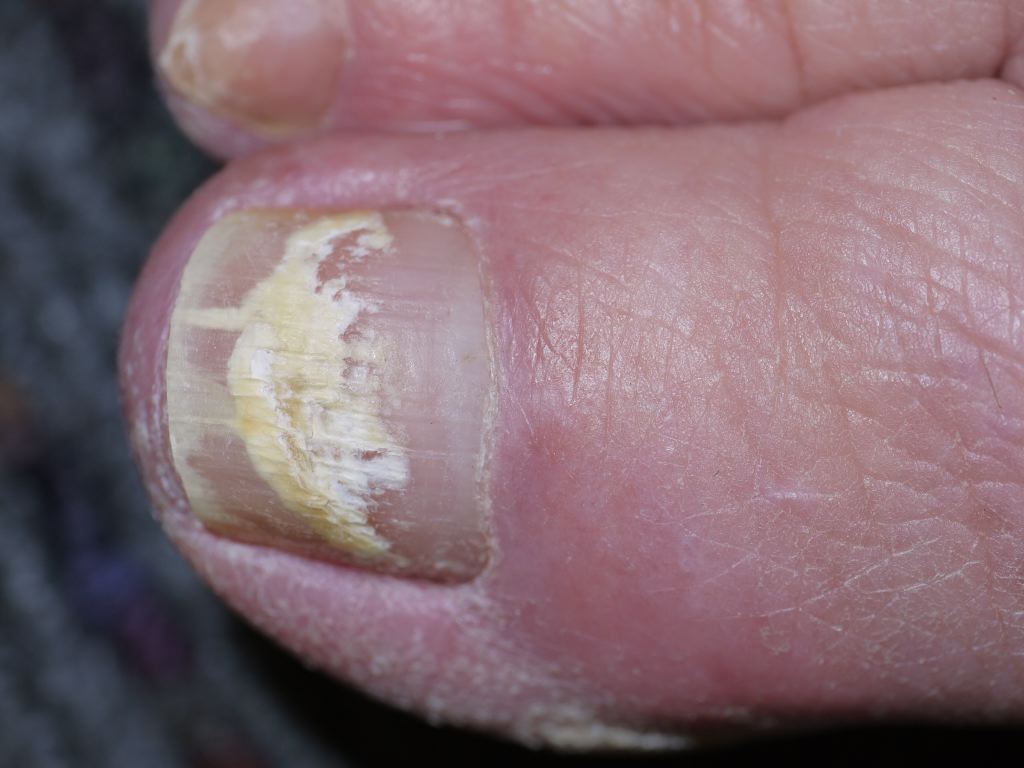
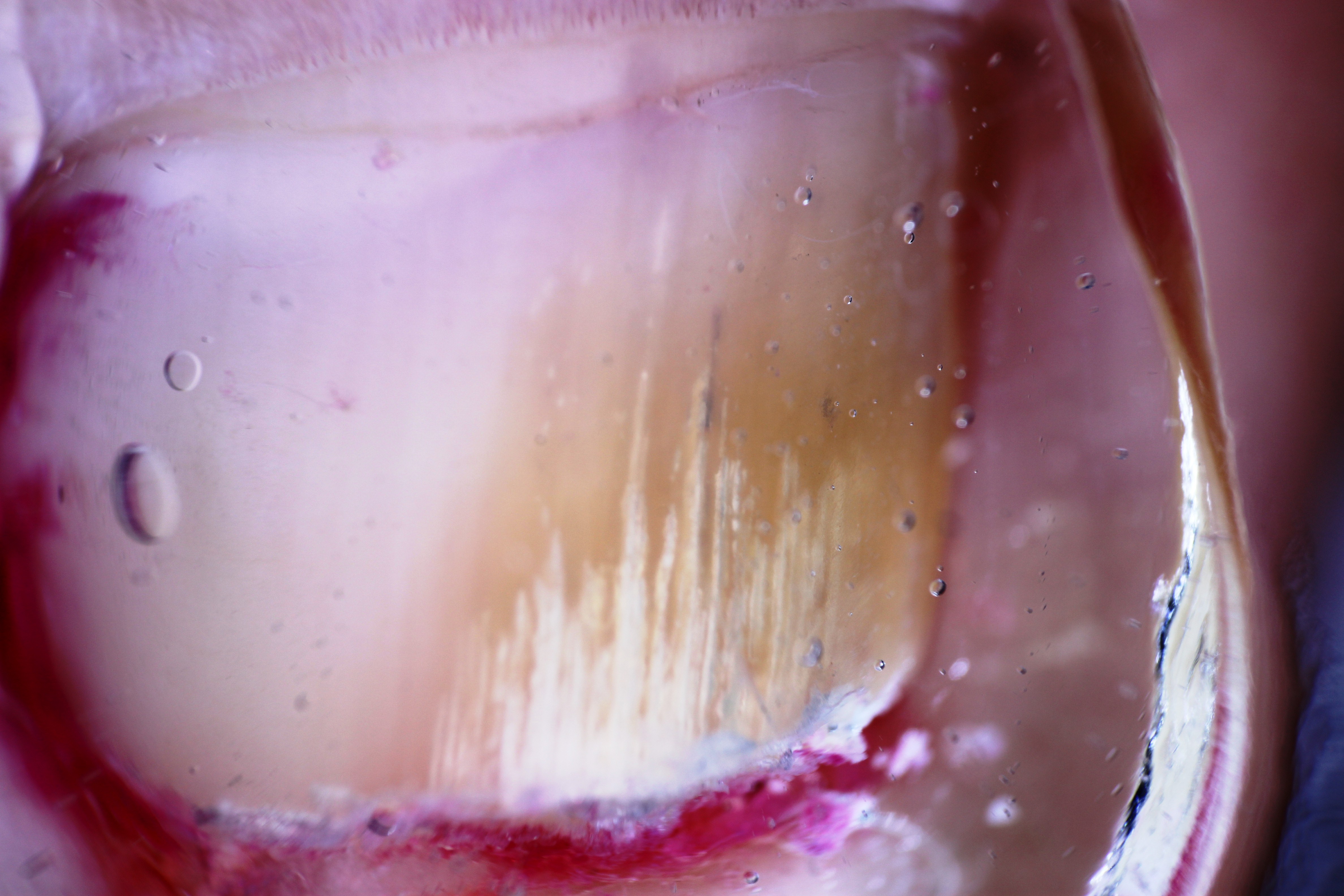
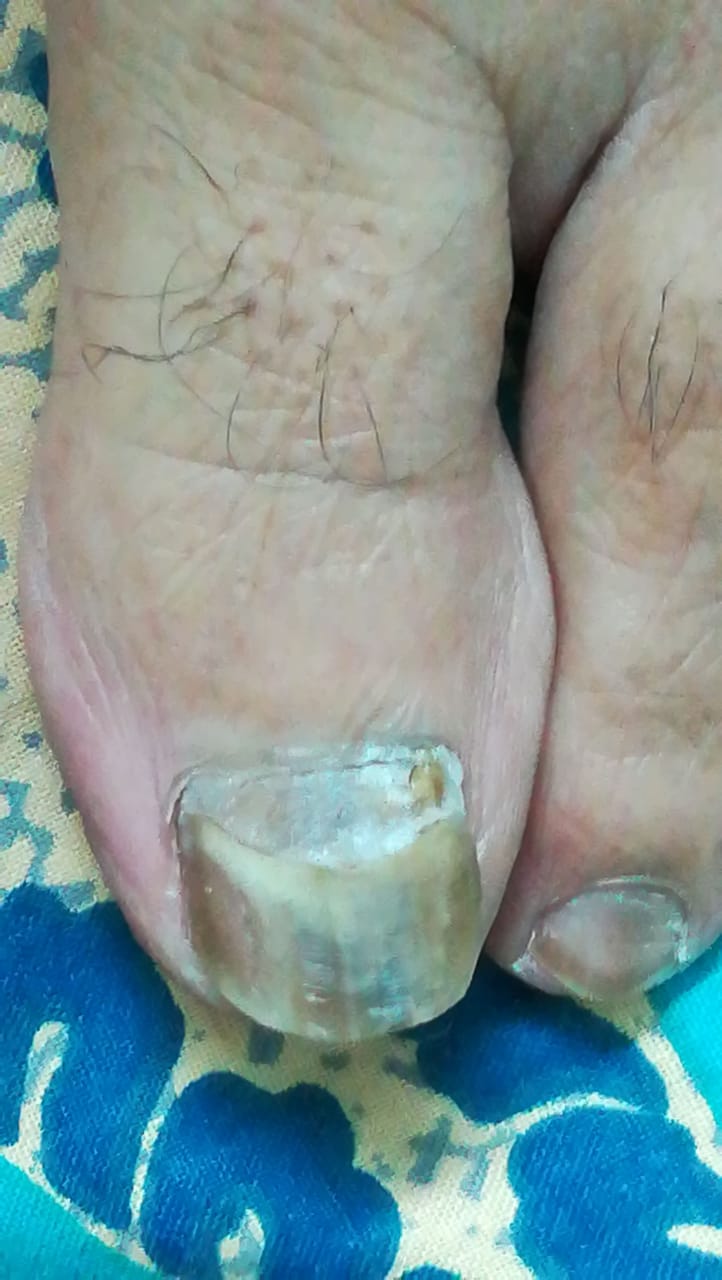
[1]
Asz-Sigall D, Tosti A, Arenas R. Tinea Unguium: Diagnosis and Treatment in Practice. Mycopathologia. 2017 Feb:182(1-2):95-100. doi: 10.1007/s11046-016-0078-4. Epub 2016 Oct 27
[PubMed PMID: 27787643]
[2]
Karaman BFO, Açıkalın A, Ünal İ, Aksungur VL. Diagnostic values of KOH examination, histological examination, and culture for onychomycosis: a latent class analysis. International journal of dermatology. 2019 Mar:58(3):319-324. doi: 10.1111/ijd.14255. Epub 2018 Sep 23
[PubMed PMID: 30246397]
[3]
Haghani I, Shams-Ghahfarokhi M, Dalimi Asl A, Shokohi T, Hedayati MT. Molecular identification and antifungal susceptibility of clinical fungal isolates from onychomycosis (uncommon and emerging species). Mycoses. 2019 Feb:62(2):128-143. doi: 10.1111/myc.12854. Epub 2018 Nov 13
[PubMed PMID: 30255665]
[4]
Becker BA, Childress MA. Common Foot Problems: Over-the-Counter Treatments and Home Care. American family physician. 2018 Sep 1:98(5):298-303
[PubMed PMID: 30216025]
[5]
Appelt L, Nenoff P, Uhrlaß S, Krüger C, Kühn P, Eichhorn K, Buder S, Beissert S, Abraham S, Aschoff R, Bauer A. [Terbinafine-resistant dermatophytoses and onychomycosis due to Trichophyton rubrum]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2021 Oct:72(10):868-877. doi: 10.1007/s00105-021-04879-1. Epub 2021 Aug 30
[PubMed PMID: 34459941]
[6]
Pote ST, Khan U, Lahiri KK, Patole MS, Thakar MR, Shah SR. Onychomycosis due to Achaetomium strumarium. Journal de mycologie medicale. 2018 Sep:28(3):510-513. doi: 10.1016/j.mycmed.2018.07.002. Epub 2018 Aug 10
[PubMed PMID: 30104134]
[7]
Youssef AB, Kallel A, Azaiz Z, Jemel S, Bada N, Chouchen A, Belhadj-Salah N, Fakhfakh N, Belhadj S, Kallel K. Onychomycosis: Which fungal species are involved? Experience of the Laboratory of Parasitology-Mycology of the Rabta Hospital of Tunis. Journal de mycologie medicale. 2018 Dec:28(4):651-654. doi: 10.1016/j.mycmed.2018.07.005. Epub 2018 Aug 11
[PubMed PMID: 30107987]
[8]
Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st Century: An Update on Diagnosis, Epidemiology, and Treatment. Journal of cutaneous medicine and surgery. 2017 Nov/Dec:21(6):525-539. doi: 10.1177/1203475417716362. Epub 2017 Jun 22
[PubMed PMID: 28639462]
[9]
Arsenijević VA, Denning DW. Estimated Burden of Serious Fungal Diseases in Serbia. Journal of fungi (Basel, Switzerland). 2018 Jun 25:4(3):. doi: 10.3390/jof4030076. Epub 2018 Jun 25
[PubMed PMID: 29941824]
[10]
Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. Journal of the American Academy of Dermatology. 2019 Apr:80(4):835-851. doi: 10.1016/j.jaad.2018.03.062. Epub 2018 Jun 28
[PubMed PMID: 29959961]
Level 3 (low-level) evidence
[11]
Aragón-Sánchez J, López-Valverde ME, Víquez-Molina G, Milagro-Beamonte A, Torres-Sopena L. Onychomycosis and Tinea Pedis in the Feet of Patients With Diabetes. The international journal of lower extremity wounds. 2023 Jun:22(2):321-327. doi: 10.1177/15347346211009409. Epub 2021 Apr 23
[PubMed PMID: 33891512]
[12]
Drago L, Micali G, Papini M, Piraccini BM, Veraldi S. Management of mycoses in daily practice. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2017 Dec:152(6):642-650. doi: 10.23736/S0392-0488.17.05683-8. Epub
[PubMed PMID: 29050446]
[13]
Maddy AJ, Tosti A. Hair and nail diseases in the mature patient. Clinics in dermatology. 2018 Mar-Apr:36(2):159-166. doi: 10.1016/j.clindermatol.2017.10.007. Epub 2017 Oct 3
[PubMed PMID: 29566920]
[14]
Zaikovska O, Pilmane M, Kisis J. Morphopathological aspects of healthy nails and nails affected by onychomycosis. Mycoses. 2014 Sep:57(9):531-6. doi: 10.1111/myc.12191. Epub 2014 Mar 24
[PubMed PMID: 24661598]
[15]
Vlahovic TC, Garcia M, Wotring K. Dermoscopy of Onychomycosis for the Podiatrist. Clinics in podiatric medicine and surgery. 2021 Oct:38(4):505-511. doi: 10.1016/j.cpm.2021.06.006. Epub
[PubMed PMID: 34538427]
[16]
Nikitha S, Kondraganti N, Kandi V. Total Dystrophic Onychomycosis of All the Nails Caused by Non-dermatophyte Fungal Species: A Case Report. Cureus. 2022 Sep:14(9):e29765. doi: 10.7759/cureus.29765. Epub 2022 Sep 29
[PubMed PMID: 36324367]
Level 3 (low-level) evidence
[17]
Gupta AK, Studholme C. Novel investigational therapies for onychomycosis: an update. Expert opinion on investigational drugs. 2016:25(3):297-305. doi: 10.1517/13543784.2016.1142529. Epub
[PubMed PMID: 26765142]
Level 3 (low-level) evidence
[18]
Daggett C, Brodell RT, Daniel CR, Jackson J. Onychomycosis in Athletes. American journal of clinical dermatology. 2019 Oct:20(5):691-698. doi: 10.1007/s40257-019-00448-4. Epub
[PubMed PMID: 31111408]
[19]
Yadav P, Singal A, Pandhi D, Das S. Clinico-mycological study of dermatophyte toenail onychomycosis in new delhi, India. Indian journal of dermatology. 2015 Mar-Apr:60(2):153-8. doi: 10.4103/0019-5154.152511. Epub
[PubMed PMID: 25814703]
[20]
Grover C, Jakhar D, Sharma S. The grid pattern of white superficial onychomycosis. Indian journal of dermatology, venereology and leprology. 2020 Sep-Oct:86(5):568-570. doi: 10.4103/ijdvl.IJDVL_699_19. Epub
[PubMed PMID: 32769307]
[21]
Almeida HL Jr, Boabaid RO, Timm V, Silva RM, Castro LA. Scanning electron microscopy of superficial white onychomycosis. Anais brasileiros de dermatologia. 2015 Sep-Oct:90(5):753-5. doi: 10.1590/abd1806-4841.20154136. Epub
[PubMed PMID: 26560225]
[22]
Mehta M, Sharma J, Bhardwaj SB. Proximal subungual onychomycosis of digitus minimus due to Aspergillus brasiliensis. The Pan African medical journal. 2020:35():79. doi: 10.11604/pamj.2020.35.79.20762. Epub 2020 Mar 19
[PubMed PMID: 32537082]
[23]
Bunyaratavej S, Bunyaratavej S, Muanprasart C, Matthapan L, Varothai S, Tangjaturonrusamee C, Pattanaprichakul P. Endonyx onychomycosis caused by Trichophyton tonsurans. Indian journal of dermatology, venereology and leprology. 2015 Jul-Aug:81(4):390-2. doi: 10.4103/0378-6323.157460. Epub
[PubMed PMID: 25994895]
[24]
Hay R. Therapy of Skin, Hair and Nail Fungal Infections. Journal of fungi (Basel, Switzerland). 2018 Aug 20:4(3):. doi: 10.3390/jof4030099. Epub 2018 Aug 20
[PubMed PMID: 30127244]
[25]
Hayette MP, Seidel L, Adjetey C, Darfouf R, Wéry M, Boreux R, Sacheli R, Melin P, Arrese J. Clinical evaluation of the DermaGenius® Nail real-time PCR assay for the detection of dermatophytes and Candida albicans in nails. Medical mycology. 2019 Apr 1:57(3):277-283. doi: 10.1093/mmy/myy020. Epub
[PubMed PMID: 29762721]
[26]
Chetana K, Menon R, David BG. Onychoscopic evaluation of onychomycosis in a tertiary care teaching hospital: a cross-sectional study from South India. International journal of dermatology. 2018 Jul:57(7):837-842. doi: 10.1111/ijd.14008. Epub 2018 Apr 26
[PubMed PMID: 29700806]
Level 2 (mid-level) evidence
[27]
Gupta AK, Hall DC, Cooper EA, Ghannoum MA. Diagnosing Onychomycosis: What's New? Journal of fungi (Basel, Switzerland). 2022 Apr 29:8(5):. doi: 10.3390/jof8050464. Epub 2022 Apr 29
[PubMed PMID: 35628720]
[28]
Pospischil I, Reinhardt C, Bontems O, Salamin K, Fratti M, Blanchard G, Chang YT, Wagner H, Hermann P, Monod M, Hoetzenecker W, Guenova E. Identification of Dermatophyte and Non-Dermatophyte Agents in Onychomycosis by PCR and DNA Sequencing-A Retrospective Comparison of Diagnostic Tools. Journal of fungi (Basel, Switzerland). 2022 Sep 27:8(10):. doi: 10.3390/jof8101019. Epub 2022 Sep 27
[PubMed PMID: 36294584]
Level 2 (mid-level) evidence
[29]
Litaiem N, Mnif E, Zeglaoui F. Dermoscopy of Onychomycosis: A Systematic Review. Dermatology practical & conceptual. 2023 Jan 1:13(1):. doi: 10.5826/dpc.1301a72. Epub 2023 Jan 1
[PubMed PMID: 36892372]
Level 1 (high-level) evidence
[30]
Lok C. [What's new in clinical dermatology?]. Annales de dermatologie et de venereologie. 2016 Dec:143 Suppl 3():S1-S10. doi: 10.1016/S0151-9638(18)30043-7. Epub
[PubMed PMID: 29429503]
[31]
Wollina U, Nenoff P, Haroske G, Haenssle HA. The Diagnosis and Treatment of Nail Disorders. Deutsches Arzteblatt international. 2016 Jul 25:113(29-30):509-18. doi: 10.3238/arztebl.2016.0509. Epub
[PubMed PMID: 27545710]
[32]
Aggarwal R, Targhotra M, Kumar B, Sahoo PK, Chauhan MK. Treatment and management strategies of onychomycosis. Journal de mycologie medicale. 2020 Jun:30(2):100949. doi: 10.1016/j.mycmed.2020.100949. Epub 2020 Mar 14
[PubMed PMID: 32234349]
[33]
Gupta AK, Talukder M. Efinaconazole in Onychomycosis. American journal of clinical dermatology. 2022 Mar:23(2):207-218. doi: 10.1007/s40257-021-00660-1. Epub 2021 Dec 13
[PubMed PMID: 34902110]
[34]
Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: Rapid Evidence Review. American family physician. 2021 Oct 1:104(4):359-367
[PubMed PMID: 34652111]
[35]
Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. Journal of the American Academy of Dermatology. 2021 Feb:84(2):497-499. doi: 10.1016/j.jaad.2020.04.172. Epub 2020 May 7
[PubMed PMID: 32387655]
Level 2 (mid-level) evidence
[36]
Lipner SR, Joseph WS, Vlahovic TC, Scher RK, Rich P, Ghannoum M, Daniel CR, Elewski B. Therapeutic Recommendations for the Treatment of Toenail Onychomycosis in the US. Journal of drugs in dermatology : JDD. 2021 Oct 1:20(10):1076-1084. doi: 10.36849/JDD.6291. Epub
[PubMed PMID: 34636509]
[37]
Ricardo JW, Lipner SR. Safety of current therapies for onychomycosis. Expert opinion on drug safety. 2020 Nov:19(11):1395-1408. doi: 10.1080/14740338.2020.1829592. Epub 2020 Oct 12
[PubMed PMID: 32990062]
Level 3 (low-level) evidence
[38]
Lipner SR, Scher RK. Onychomycosis: Treatment and prevention of recurrence. Journal of the American Academy of Dermatology. 2019 Apr:80(4):853-867. doi: 10.1016/j.jaad.2018.05.1260. Epub 2018 Jun 28
[PubMed PMID: 29959962]
[39]
Hanna S, Andriessen A, Beecker J, Gilbert M, Goldstein E, Kalia S, King A, Kraft J, Lynde C, Singh D, Turchin I, Zip C. Clinical Insights About Onychomycosis and Its Treatment: A Consensus. Journal of drugs in dermatology : JDD. 2018 Mar 1:17(3):253-262
[PubMed PMID: 29537443]
Level 3 (low-level) evidence
[40]
Zeichner JA. Onychomycosis to Fungal Superinfection: Prevention Strategies and Considerations. Journal of drugs in dermatology : JDD. 2015 Oct:14(10 Suppl):s32-4
[PubMed PMID: 26461832]
[41]
Shemer A, Gupta AK, Babaev M, Barzilai A, Farhi R, Daniel Iii CR. A Retrospective Study Comparing K101 Nail Solution as a Monotherapy and in Combination with Oral Terbinafine or Itraconazole for the Treatment of Toenail Onychomycosis. Skin appendage disorders. 2018 Aug:4(3):166-170. doi: 10.1159/000484211. Epub 2017 Nov 16
[PubMed PMID: 30197895]
Level 2 (mid-level) evidence
[42]
Salakshna N, Bunyaratavej S, Matthapan L, Lertrujiwanit K, Leeyaphan C. A cohort study of risk factors, clinical presentations, and outcomes for dermatophyte, nondermatophyte, and mixed toenail infections. Journal of the American Academy of Dermatology. 2018 Dec:79(6):1145-1146. doi: 10.1016/j.jaad.2018.05.041. Epub 2018 May 31
[PubMed PMID: 29860042]