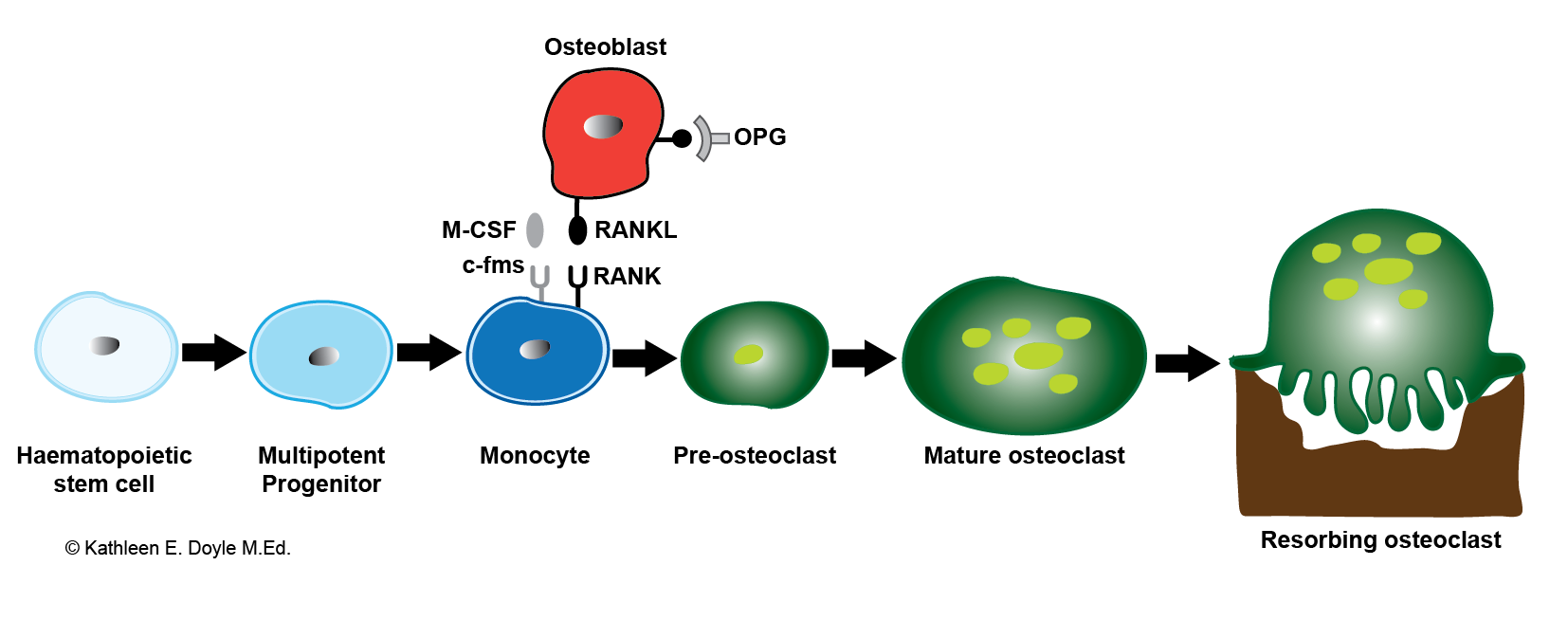
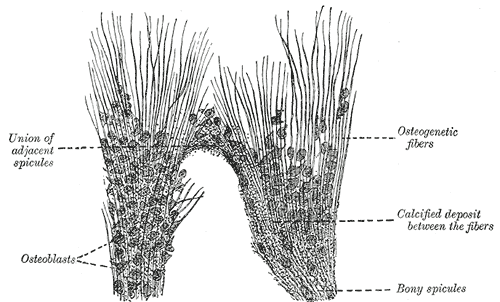
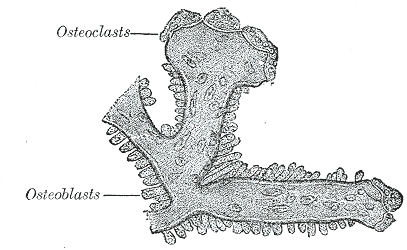
[1]
Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Current drug targets. Inflammation and allergy. 2005 Jun:4(3):325-8
[PubMed PMID: 16101541]
[2]
Morello R. Osteogenesis imperfecta and therapeutics. Matrix biology : journal of the International Society for Matrix Biology. 2018 Oct:71-72():294-312. doi: 10.1016/j.matbio.2018.03.010. Epub 2018 Mar 11
[PubMed PMID: 29540309]
[3]
Feng X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Current chemical biology. 2009 May 1:3(2):189-196
[PubMed PMID: 20161446]
[4]
Bou Assaf R, Zibara K, Fayyad-Kazan M, Al-Nemer F, Cordahi M, Khairallah S, Badran B, Berbéri A. Healing of Bone Defects in Pig's Femur Using Mesenchymal Cells Originated from the Sinus Membrane with Different Scaffolds. Stem cells international. 2019:2019():4185942. doi: 10.1155/2019/4185942. Epub 2019 Sep 30
[PubMed PMID: 31662765]
[5]
Le BQ, Nurcombe V, Cool SM, van Blitterswijk CA, de Boer J, LaPointe VLS. The Components of Bone and What They Can Teach Us about Regeneration. Materials (Basel, Switzerland). 2017 Dec 22:11(1):. doi: 10.3390/ma11010014. Epub 2017 Dec 22
[PubMed PMID: 29271933]
[6]
Ottewell PD. The role of osteoblasts in bone metastasis. Journal of bone oncology. 2016 Sep:5(3):124-127
[PubMed PMID: 27761372]
[7]
Matsuo K, Irie N. Osteoclast-osteoblast communication. Archives of biochemistry and biophysics. 2008 May 15:473(2):201-9. doi: 10.1016/j.abb.2008.03.027. Epub 2008 Mar 29
[PubMed PMID: 18406338]
[8]
Rutkovskiy A, Stensløkken KO, Vaage IJ. Osteoblast Differentiation at a Glance. Medical science monitor basic research. 2016 Sep 26:22():95-106
[PubMed PMID: 27667570]
[9]
Roeder E, Matthews BG, Kalajzic I. Visual reporters for study of the osteoblast lineage. Bone. 2016 Nov:92():189-195. doi: 10.1016/j.bone.2016.09.004. Epub 2016 Sep 8
[PubMed PMID: 27616604]
[10]
Rosenberg N, Rosenberg O, Soudry M. Osteoblasts in bone physiology-mini review. Rambam Maimonides medical journal. 2012 Apr:3(2):e0013. doi: 10.5041/RMMJ.10080. Epub 2012 Apr 30
[PubMed PMID: 23908837]
[11]
Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed research international. 2015:2015():421746. doi: 10.1155/2015/421746. Epub 2015 Jul 13
[PubMed PMID: 26247020]
[12]
Marie PJ, Cohen-Solal M. The Expanding Life and Functions of Osteogenic Cells: From Simple Bone-Making Cells to Multifunctional Cells and Beyond. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018 Feb:33(2):199-210. doi: 10.1002/jbmr.3356. Epub 2018 Jan 16
[PubMed PMID: 29206311]
[13]
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connective tissue research. 2018 Mar:59(2):99-107. doi: 10.1080/03008207.2017.1290085. Epub 2017 Mar 21
[PubMed PMID: 28324674]
[14]
Czekanska EM, Stoddart MJ, Richards RG, Hayes JS. In search of an osteoblast cell model for in vitro research. European cells & materials. 2012 Jul 9:24():1-17
[PubMed PMID: 22777949]
[15]
Akkiraju H, Bonor J, Nohe A. An Improved Immunostaining and Imaging Methodology to Determine Cell and Protein Distributions within the Bone Environment. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2016 Mar:64(3):168-78. doi: 10.1369/0022155415626765. Epub 2015 Dec 30
[PubMed PMID: 26718242]
[16]
Sridharan G, Shankar AA. Toluidine blue: A review of its chemistry and clinical utility. Journal of oral and maxillofacial pathology : JOMFP. 2012 May:16(2):251-5. doi: 10.4103/0973-029X.99081. Epub
[PubMed PMID: 22923899]
[17]
Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian journal of clinical biochemistry : IJCB. 2014 Jul:29(3):269-78. doi: 10.1007/s12291-013-0408-y. Epub 2013 Nov 26
[PubMed PMID: 24966474]
Level 3 (low-level) evidence
[18]
Cosby CN, Troiano NW, Kacena MA. The Effects of Storage Conditions on the Preservation of Enzymatic Activity in Bone. Journal of histotechnology. 2008 Dec:31(4):169-173
[PubMed PMID: 20686670]
[19]
Barger A, Graca R, Bailey K, Messick J, de Lorimier LP, Fan T, Hoffmann W. Use of alkaline phosphatase staining to differentiate canine osteosarcoma from other vimentin-positive tumors. Veterinary pathology. 2005 Mar:42(2):161-5
[PubMed PMID: 15753469]
[20]
Lee JM, Kim MG, Byun JH, Kim GC, Ro JH, Hwang DS, Choi BB, Park GC, Kim UK. The effect of biomechanical stimulation on osteoblast differentiation of human jaw periosteum-derived stem cells. Maxillofacial plastic and reconstructive surgery. 2017 Dec:39(1):7. doi: 10.1186/s40902-017-0104-6. Epub 2017 Mar 5
[PubMed PMID: 28303237]
[22]
Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW. Scanning electron microscopy. Current protocols in microbiology. 2012 May:Chapter 2():Unit 2B.2.. doi: 10.1002/9780471729259.mc02b02s25. Epub
[PubMed PMID: 22549162]
[23]
Ornoy A, Atkin I, Levy J. Ultrastructural studies on the origin and structure of matrix vesicles in bone of young rats. Acta anatomica. 1980:106(4):450-61
[PubMed PMID: 7386166]
[24]
Shah FA, Ruscsák K, Palmquist A. 50 years of scanning electron microscopy of bone-a comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone research. 2019:7():15. doi: 10.1038/s41413-019-0053-z. Epub 2019 May 22
[PubMed PMID: 31123620]
Level 3 (low-level) evidence
[25]
Winey M, Meehl JB, O'Toole ET, Giddings TH Jr. Conventional transmission electron microscopy. Molecular biology of the cell. 2014 Feb:25(3):319-23. doi: 10.1091/mbc.E12-12-0863. Epub
[PubMed PMID: 24482357]
[26]
Bottini M, Mebarek S, Anderson KL, Strzelecka-Kiliszek A, Bozycki L, Simão AMS, Bolean M, Ciancaglini P, Pikula JB, Pikula S, Magne D, Volkmann N, Hanein D, Millán JL, Buchet R. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochimica et biophysica acta. General subjects. 2018 Mar:1862(3):532-546. doi: 10.1016/j.bbagen.2017.11.005. Epub 2017 Nov 3
[PubMed PMID: 29108957]
[27]
Clarke B. Normal bone anatomy and physiology. Clinical journal of the American Society of Nephrology : CJASN. 2008 Nov:3 Suppl 3(Suppl 3):S131-9. doi: 10.2215/CJN.04151206. Epub
[PubMed PMID: 18988698]
[28]
Małkiewicz A, Dziedzic M. Bone marrow reconversion - imaging of physiological changes in bone marrow. Polish journal of radiology. 2012 Oct:77(4):45-50
[PubMed PMID: 23269936]
[29]
Gurevitch O, Slavin S, Feldman AG. Conversion of red bone marrow into yellow - Cause and mechanisms. Medical hypotheses. 2007:69(3):531-6
[PubMed PMID: 17433565]
[30]
Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Therapeutic advances in musculoskeletal disease. 2016 Dec:8(6):225-235. doi: 10.1177/1759720X16670154. Epub 2016 Oct 5
[PubMed PMID: 28255336]
Level 3 (low-level) evidence
[31]
Delgado-Calle J, Bellido T. Osteocytes and Skeletal Pathophysiology. Current molecular biology reports. 2015 Dec:1(4):157-167
[PubMed PMID: 26693137]
[32]
Caetano-Lopes J, Canhão H, Fonseca JE. Osteoblasts and bone formation. Acta reumatologica portuguesa. 2007 Apr-Jun:32(2):103-10
[PubMed PMID: 17572649]
[33]
Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. Journal of cellular biochemistry. 2013 May:114(5):975-84. doi: 10.1002/jcb.24439. Epub
[PubMed PMID: 23225263]
[34]
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997 May 30:89(5):747-54
[PubMed PMID: 9182762]
[36]
Jia D, Heersche JN. Insulin-like growth factor-1 and -2 stimulate osteoprogenitor proliferation and differentiation and adipocyte formation in cell populations derived from adult rat bone. Bone. 2000 Dec:27(6):785-94
[PubMed PMID: 11113389]
[37]
Bellido T, Borba VZ, Roberson P, Manolagas SC. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997 Sep:138(9):3666-76
[PubMed PMID: 9275051]
[38]
Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Frontiers in bioscience : a journal and virtual library. 2007 May 1:12():3068-92
[PubMed PMID: 17485283]
[39]
Yamashita T, Takahashi N, Udagawa N. New roles of osteoblasts involved in osteoclast differentiation. World journal of orthopedics. 2012 Nov 18:3(11):175-81. doi: 10.5312/wjo.v3.i11.175. Epub
[PubMed PMID: 23330072]
[40]
Feng X, Teitelbaum SL. Osteoclasts: New Insights. Bone research. 2013 Mar:1(1):11-26. doi: 10.4248/BR201301003. Epub 2013 Mar 29
[PubMed PMID: 26273491]
[41]
Zhong Z, Ethen NJ, Williams BO. WNT signaling in bone development and homeostasis. Wiley interdisciplinary reviews. Developmental biology. 2014 Nov-Dec:3(6):489-500. doi: 10.1002/wdev.159. Epub 2014 Sep 30
[PubMed PMID: 25270716]
[42]
Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends in endocrinology and metabolism: TEM. 2013 Jan:24(1):31-9. doi: 10.1016/j.tem.2012.10.003. Epub
[PubMed PMID: 23245947]
[43]
Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. The Journal of clinical investigation. 2006 May:116(5):1202-9
[PubMed PMID: 16670761]
[44]
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009 Jul:17(1):9-26. doi: 10.1016/j.devcel.2009.06.016. Epub
[PubMed PMID: 19619488]
[45]
Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cellular signalling. 2009 Aug:21(8):1245-54. doi: 10.1016/j.cellsig.2009.02.012. Epub 2009 Feb 26
[PubMed PMID: 19249350]
[46]
Wein MN. Parathyroid Hormone Signaling in Osteocytes. JBMR plus. 2018 Jan:2(1):22-30. doi: 10.1002/jbm4.10021. Epub 2017 Nov 10
[PubMed PMID: 30283888]
[47]
Jilka RL, O'Brien CA, Bartell SM, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Nov:25(11):2427-37. doi: 10.1002/jbmr.145. Epub
[PubMed PMID: 20533302]
[48]
Lee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Current opinion in nephrology and hypertension. 2009 Jul:18(4):298-302. doi: 10.1097/MNH.0b013e32832c2264. Epub
[PubMed PMID: 19395963]
Level 3 (low-level) evidence
[49]
Damasiewicz MJ, Nickolas TL. Rethinking Bone Disease in Kidney Disease. JBMR plus. 2018 Nov:2(6):309-322. doi: 10.1002/jbm4.10117. Epub 2018 Nov 15
[PubMed PMID: 30460334]
[51]
Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bilezikian JP. Bone disease in primary hyperparathyroidism. Arquivos brasileiros de endocrinologia e metabologia. 2014 Jul:58(5):553-61
[PubMed PMID: 25166047]
[52]
Wheater G, Elshahaly M, Tuck SP, Datta HK, van Laar JM. The clinical utility of bone marker measurements in osteoporosis. Journal of translational medicine. 2013 Aug 29:11():201. doi: 10.1186/1479-5876-11-201. Epub 2013 Aug 29
[PubMed PMID: 23984630]
[53]
Rogers A, Eastell R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: clinical utility in metabolic bone disease assessment. The Journal of clinical endocrinology and metabolism. 2005 Nov:90(11):6323-31
[PubMed PMID: 16105967]
[54]
Bhattoa HP. Laboratory aspects and clinical utility of bone turnover markers. EJIFCC. 2018 Jul:29(2):117-128
[PubMed PMID: 30050395]
[55]
Camozzi V, Tossi A, Simoni E, Pagani F, Francucci CM, Moro L. Role of biochemical markers of bone remodeling in clinical practice. Journal of endocrinological investigation. 2007:30(6 Suppl):13-7
[PubMed PMID: 17721068]
[57]
Walker HK, Hall WD, Hurst JW, Vroon DH, Israili Z. Alkaline Phosphatase and Gamma Glutamyltransferase. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:():
[PubMed PMID: 21250047]
[58]
Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. The Journal of clinical investigation. 1991 Jul:88(1):62-6
[PubMed PMID: 2056131]
[59]
Siddique A, Kowdley KV. Approach to a patient with elevated serum alkaline phosphatase. Clinics in liver disease. 2012 May:16(2):199-229. doi: 10.1016/j.cld.2012.03.012. Epub 2012 Apr 6
[PubMed PMID: 22541695]
[60]
Fu R, Peng F, Liu H, Wang Y, Li L, Wang G, Song J, Shao Z. Clinical significance of osteoblast precursors and osteoclast precursors in earlier diagnosis and monitoring of myeloma bone disease. Annals of hematology. 2016 Jun:95(7):1099-106. doi: 10.1007/s00277-016-2657-3. Epub 2016 Apr 27
[PubMed PMID: 27118542]
[61]
Feng X, McDonald JM. Disorders of bone remodeling. Annual review of pathology. 2011:6():121-45. doi: 10.1146/annurev-pathol-011110-130203. Epub
[PubMed PMID: 20936937]
[62]
Smellie WS, Forth J, Ryder S, Galloway MJ, Wood AC, Watson ID. Best practice in primary care pathology: review 5. Journal of clinical pathology. 2006 Dec:59(12):1229-37
[PubMed PMID: 16644875]
[63]
Deeb A, Elfatih A. Could Alerting Physicians for Low Alkaline Phosphatase Levels Be Helpful in Early Diagnosis of Hypophosphatasia? Journal of clinical research in pediatric endocrinology. 2018 Mar 1:10(1):19-24. doi: 10.4274/jcrpe.4426. Epub 2017 Aug 2
[PubMed PMID: 28766503]
[64]
Morava E, Kárteszi J, Weisenbach J, Caliebe A, Mundlos S, Méhes K. Cleidocranial dysplasia with decreased bone density and biochemical findings of hypophosphatasia. European journal of pediatrics. 2002 Nov:161(11):619-22
[PubMed PMID: 12424590]
[65]
Agustina H, Asyifa I, Aziz A, Hernowo BS. The Role of Osteocalcin and Alkaline Phosphatase Immunohistochemistry in Osteosarcoma Diagnosis. Pathology research international. 2018:2018():6346409. doi: 10.1155/2018/6346409. Epub 2018 May 3
[PubMed PMID: 29854380]
[66]
Aparisi T, Arborgh B, Ericsson JL. Giant cell tumor of bone. Fine structural localization of alkaline phosphatase. Virchows Archiv. A, Pathological anatomy and histology. 1978 Jul 26:378(4):287-95
[PubMed PMID: 150116]
[68]
Izumi Y. Alkaline phosphatase as a biochemical maturity index in female adolescence. Spine. 1993 Nov:18(15):2257-60
[PubMed PMID: 8278842]
[69]
Blumsohn A, Hannon RA, Wrate R, Barton J, al-Dehaimi AW, Colwell A, Eastell R. Biochemical markers of bone turnover in girls during puberty. Clinical endocrinology. 1994 May:40(5):663-70
[PubMed PMID: 7516828]
[70]
Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud publica de Mexico. 2009:51 Suppl 1():S5-17
[PubMed PMID: 19287894]
[71]
Lu J, Shin Y, Yen MS, Sun SS. Peak Bone Mass and Patterns of Change in Total Bone Mineral Density and Bone Mineral Contents From Childhood Into Young Adulthood. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2016 Apr-Jun:19(2):180-91. doi: 10.1016/j.jocd.2014.08.001. Epub 2014 Oct 18
[PubMed PMID: 25440183]
[72]
Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. Journal of biomechanics. 2010 Jan 5:43(1):108-18. doi: 10.1016/j.jbiomech.2009.09.016. Epub 2009 Oct 8
[PubMed PMID: 19818443]
[73]
Teichtahl AJ, Wluka AE, Wijethilake P, Wang Y, Ghasem-Zadeh A, Cicuttini FM. Wolff's law in action: a mechanism for early knee osteoarthritis. Arthritis research & therapy. 2015 Sep 1:17(1):207. doi: 10.1186/s13075-015-0738-7. Epub 2015 Sep 1
[PubMed PMID: 26324398]
[74]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Bikle DD. Vitamin D: Production, Metabolism and Mechanisms of Action. Endotext. 2000:():
[PubMed PMID: 25905172]
[75]
Chapuy MC, Durr F, Chapuy P. Age-related changes in parathyroid hormone and 25 hydroxycholecalciferol levels. Journal of gerontology. 1983 Jan:38(1):19-22
[PubMed PMID: 6600237]
[76]
Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. European journal of rheumatology. 2017 Mar:4(1):46-56. doi: 10.5152/eurjrheum.2016.048. Epub 2016 Dec 30
[PubMed PMID: 28293453]
Level 3 (low-level) evidence
[78]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Rosen CJ. The Epidemiology and Pathogenesis of Osteoporosis. Endotext. 2000:():
[PubMed PMID: 25905357]
[79]
Akkawi I, Zmerly H. Osteoporosis: Current Concepts. Joints. 2018 Jun:6(2):122-127. doi: 10.1055/s-0038-1660790. Epub 2018 Jun 14
[PubMed PMID: 30051110]
[80]
Sheu A, Diamond T. Bone mineral density: testing for osteoporosis. Australian prescriber. 2016 Apr:39(2):35-9. doi: 10.18773/austprescr.2016.020. Epub 2016 Apr 1
[PubMed PMID: 27340320]
[81]
Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. European journal of endocrinology. 2015 Sep:173(3):R131-51. doi: 10.1530/EJE-15-0118. Epub 2015 May 13
[PubMed PMID: 25971649]
[82]
Rosen CJ. Endocrine disorders and osteoporosis. Current opinion in rheumatology. 1997 Jul:9(4):355-61
[PubMed PMID: 9229183]
Level 3 (low-level) evidence
[83]
Robinson LJ, Yaroslavskiy BB, Griswold RD, Zadorozny EV, Guo L, Tourkova IL, Blair HC. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Experimental cell research. 2009 Apr 15:315(7):1287-301. doi: 10.1016/j.yexcr.2009.01.014. Epub 2009 Jan 30
[PubMed PMID: 19331827]
[84]
Xiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Mar:27(3):499-505. doi: 10.1002/jbmr.1547. Epub
[PubMed PMID: 22354849]
[85]
Yasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World journal of orthopedics. 2013 Oct 18:4(4):207-17. doi: 10.5312/wjo.v4.i4.207. Epub 2013 Oct 18
[PubMed PMID: 24147256]
[86]
Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003 Feb:32(2):136-41
[PubMed PMID: 12633785]
[87]
Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. The Journal of experimental medicine. 1997 Aug 18:186(4):489-95
[PubMed PMID: 9254647]
[88]
Riggs BL. The mechanisms of estrogen regulation of bone resorption. The Journal of clinical investigation. 2000 Nov:106(10):1203-4
[PubMed PMID: 11086020]
[89]
Streicher C, Heyny A, Andrukhova O, Haigl B, Slavic S, Schüler C, Kollmann K, Kantner I, Sexl V, Kleiter M, Hofbauer LC, Kostenuik PJ, Erben RG. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Scientific reports. 2017 Jul 25:7(1):6460. doi: 10.1038/s41598-017-06614-0. Epub 2017 Jul 25
[PubMed PMID: 28744019]
[90]
. Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause (New York, N.Y.). 2010 Jan-Feb:17(1):25-54; quiz 55-6. doi: 10.1097/gme.0b013e3181c617e6. Epub
[PubMed PMID: 20061894]
[91]
Cranney A, Papaioannou A, Zytaruk N, Hanley D, Adachi J, Goltzman D, Murray T, Hodsman A, Clinical Guidelines Committee of Osteoporosis Canada. Parathyroid hormone for the treatment of osteoporosis: a systematic review. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006 Jul 4:175(1):52-9
[PubMed PMID: 16818910]
Level 1 (high-level) evidence
[92]
Lou S, Lv H, Yin P, Li Z, Tang P, Wang Y. Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2019 Jan:30(1):59-70. doi: 10.1007/s00198-018-4790-4. Epub 2018 Dec 11
[PubMed PMID: 30539271]
Level 1 (high-level) evidence
[93]
Datta NS. Osteoporotic fracture and parathyroid hormone. World journal of orthopedics. 2011 Aug 18:2(8):67-74. doi: 10.5312/wjo.v2.i8.67. Epub
[PubMed PMID: 22474638]
[94]
Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. The Journal of clinical investigation. 2005 Sep:115(9):2322-4
[PubMed PMID: 16138187]
[95]
Byun JH, Jang S, Lee S, Park S, Yoon HK, Yoon BH, Ha YC. The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis. Journal of bone metabolism. 2017 Feb:24(1):37-49. doi: 10.11005/jbm.2017.24.1.37. Epub 2017 Feb 28
[PubMed PMID: 28326300]
Level 1 (high-level) evidence
[96]
Reid IR. Bisphosphonates in the treatment of osteoporosis: a review of their contribution and controversies. Skeletal radiology. 2011 Sep:40(9):1191-6. doi: 10.1007/s00256-011-1164-9. Epub 2011 Aug 17
[PubMed PMID: 21847749]
[97]
Maruotti N, Corrado A, Cantatore FP. Osteoblast role in osteoarthritis pathogenesis. Journal of cellular physiology. 2017 Nov:232(11):2957-2963. doi: 10.1002/jcp.25969. Epub 2017 May 24
[PubMed PMID: 28425564]
[99]
McCauley LK, Martin TJ. Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Jun:27(6):1231-9. doi: 10.1002/jbmr.1617. Epub 2012 May 1
[PubMed PMID: 22549910]
[100]
Luparello C. Parathyroid Hormone-Related Protein (PTHrP): A Key Regulator of Life/Death Decisions by Tumor Cells with Potential Clinical Applications. Cancers. 2011 Jan 20:3(1):396-407. doi: 10.3390/cancers3010396. Epub 2011 Jan 20
[PubMed PMID: 24212621]
[101]
Soki FN, Park SI, McCauley LK. The multifaceted actions of PTHrP in skeletal metastasis. Future oncology (London, England). 2012 Jul:8(7):803-17. doi: 10.2217/fon.12.76. Epub
[PubMed PMID: 22830401]
[102]
Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nature reviews. Endocrinology. 2011 Apr:7(4):208-18. doi: 10.1038/nrendo.2010.227. Epub 2011 Jan 4
[PubMed PMID: 21200394]
[103]
Shupp AB, Kolb AD, Mukhopadhyay D, Bussard KM. Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers. 2018 Jun 4:10(6):. doi: 10.3390/cancers10060182. Epub 2018 Jun 4
[PubMed PMID: 29867053]
[104]
Mannstadt M, Jüppner H, Gardella TJ. Receptors for PTH and PTHrP: their biological importance and functional properties. The American journal of physiology. 1999 Nov:277(5):F665-75. doi: 10.1152/ajprenal.1999.277.5.F665. Epub
[PubMed PMID: 10564229]
[105]
Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. The Journal of clinical investigation. 2011 Dec:121(12):4655-69. doi: 10.1172/JCI46134. Epub 2011 Nov 7
[PubMed PMID: 22056386]
[106]
Yang Y, Wang B. PTH1R-CaSR Cross Talk: New Treatment Options for Breast Cancer Osteolytic Bone Metastases. International journal of endocrinology. 2018:2018():7120979. doi: 10.1155/2018/7120979. Epub 2018 Jul 29
[PubMed PMID: 30151009]
[107]
Carter PH, Schipani E. The roles of parathyroid hormone and calcitonin in bone remodeling: prospects for novel therapeutics. Endocrine, metabolic & immune disorders drug targets. 2006 Mar:6(1):59-76
[PubMed PMID: 16611165]
[108]
Migliaccio S, Brama M, Spera G. The differential effects of bisphosphonates, SERMS (selective estrogen receptor modulators), and parathyroid hormone on bone remodeling in osteoporosis. Clinical interventions in aging. 2007:2(1):55-64
[PubMed PMID: 18044075]
[109]
Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. The Journal of clinical investigation. 2002 Apr:109(8):1041-8
[PubMed PMID: 11956241]
[110]
Becker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesthesia progress. 2013 Spring:60(1):25-31; quiz 32. doi: 10.2344/0003-3006-60.1.25. Epub
[PubMed PMID: 23506281]