Continuing Education Activity
Massive transfusion involves administering 10 or more units of whole blood or packed red blood cells (PRBCs) within 24 hours. The primary objective of a massive transfusion is to prevent fatal outcomes resulting from critical hypoperfusion while striving to attain hemostasis through surgical, endoscopic, interventional radiological, or alternative interventions. This activity reviews the contemporary literature regarding massive transfusion protocols and explores the potential complications of this life-saving intervention. This activity also highlights the crucial role of the interprofessional healthcare team in caring for patients requiring blood products.
Objectives:
Identify the criteria and indications for activating a massive transfusion protocol in emergency situations.
Implement massive transfusion protocols promptly and effectively in emergencies, following established guidelines and protocols, to initiate rapid and appropriate blood product transfusion.
Apply point-of-care coagulation testing such as thromboelastography to guide and adjust blood product transfusions, ensuring patient safety and minimizing the risk of transfusion-related complications.
Communicate effectively with the interprofessional healthcare providers and the blood bank personnel to ensure seamless coordination during the entire process of massive transfusion.
Introduction
A massive transfusion involves the administration of 10 units or more of whole blood or packed red blood cells (PRBCs) within 24 hours. An ultra-massive transfusion is defined as using more than 20 units of PRBCs within a 24- to 48-hour period. The primary objective of a massive transfusion is to prevent fatal outcomes resulting from critical hypoperfusion-related complications while striving to attain hemostasis.[1] Furthermore, this topic explores the significance of major transfusion protocols (MTPs) and the indications, contraindications, and potential complications of this life-saving intervention.[2][3][4]
Patients across medical specialties may require massive transfusions. Although cardiac and vascular surgeries are the most common scenarios that necessitate massive transfusions, other frequent causes include gastrointestinal and obstetrical hemorrhages, liver transplants, and trauma. An estimated 3% to 5% of civilian trauma patients and 10% of military trauma patients typically undergo a massive transfusion. Although massive transfusions are relatively rare, patients in need of such transfusions often experience high mortality rates.
As massive transfusions are unpredictable and require a substantial quantity of blood products over an extended duration, pre-planning among the emergency department, trauma service, surgical team, blood bank, and delivery personnel is essential. The Assessment of Blood Consumption (ABC) score is a tool for predicting the necessity of massive transfusions. Monitoring volume status, tissue oxygenation, bleeding management, coagulation abnormalities, and acid-base balance is imperative throughout a massive transfusion.[5] The development and implementation of MTPs can effectively lower mortality rates and reduce the consumption of blood products.
Indications
Any situation resulting in acute blood loss and hemodynamic instability is a potential indication of a massive transfusion. Scenarios that may lead to, but are not limited to, a massive transfusion include trauma-related bleeding, obstetrical hemorrhage, surgery, and gastrointestinal bleeding.[6][7] Efforts to mitigate the uncertainty surrounding the timing and necessity of massive transfusions by utilizing measures such as the Shock Index offer limited value.[8]
The ABC score is a clinically useful and validated scoring system based on 4 variables, which include a pulse rate exceeding 120 bpm, a systolic blood pressure below 90 mm Hg, a positive result on the Focused Assessment with Sonography for Trauma (FAST) exam, and a penetrating torso injury. Each variable is assigned 1 point, and patients with a score of 2 or more points indicate the necessity to initiate an MTP. The ABC score demonstrates a positive predictive value of 50% to 55%, implying that 45% to 50% of patients who trigger the MTP will not necessitate a massive transfusion. The ABC score identifies over 95% of patients requiring a massive transfusion but has a negative predictive value of less than 5%.
In general, the criteria that warrant activation of an MTP include:
- An ABC score of 2 or more points
- Persistent hemodynamic instability
- Active bleeding requiring surgery or angioembolization
- Blood transfusion in the trauma bay
Contraindications
There are no absolute contraindications for a massive transfusion.
Equipment
The availability of blood products and the establishment of suitable intravenous (IV) or intraosseous access are 2 essential components for a massive transfusion. According to the Hagen-Poiseuille equation, catheters with a larger diameter and shorter length will yield the highest flow rates. The flow rate is directly proportional to the fourth power of the catheter's radius and inversely proportional to the length of the catheter and the viscosity of the fluid passing through it. In most patients undergoing a massive transfusion, rapid blood replacement is imperative. Hence, it is essential to assemble and insert large-bore catheters, typically ranging from 14 to 18 gauge, into the patient, either peripherally or centrally (IV) or through intraosseous access, as clinically indicated.
Additional equipment or resources that may be necessary include:
- Effective communication with blood banks regarding the evolving situation of massive blood loss.
- Sufficient number of personnel for timely sample collection and the procurement of blood and blood products.
- A blood refrigerator within the resuscitation bay stocked with universal donor products, ideally comprising a minimum of 8 units of O-negative PRBCs and 8 units of thawed group AB or low titer anti-B group A plasma.
- Inclusion of in-line fluid warmers and surface warmers.
- Continuous core temperature monitors.
- An invasive arterial blood pressure monitor.
- Adequate supply of colloid and crystalloid infusion sets.
- Point-of-care testing for various bodily functions, including arterial blood gas (ABG), hemoglobin, electrolytes, lactate, and thromboelastography (TEG).
- Rapid infusion pumps or pressure bags to expedite the fluid infusion rates.
See Image. Autotransfusion Set Up. ATS bags and atrium.
Personnel
Massive transfusions necessitate the coordination of the emergency department, trauma and surgical teams, healthcare professionals, blood banks, and designated runners. Many institutions use an alert system similar to those used in trauma cases, which notifies the requisite personnel that a massive transfusion is imminent or in progress.
Preparation
The most effective preparation involves having an MTP in place, with immediate notification of the blood bank being of utmost importance. Swift activation of the MTP enables the blood bank to proactively prepare and deliver the necessary products before they are required. Healthcare professionals must ensure proper respiratory and cardiac monitoring for patients, as well as adequate IV or intraosseous access for blood product delivery.
Technique or Treatment
During a massive transfusion, the primary focus is to sustain cardiac output and optimize oxygen-carrying capacity. Institution-specific protocols streamline the process of ordering blood products and receiving them expeditiously from the blood bank. Although these protocols may differ among institutions, MTPs should prioritize the delivery of PRBCs along with platelets and fresh frozen plasma (FFP).[9][10][11]
At baseline, oxygen delivery to tissues is approximately 4 times the rate of tissue oxygen consumption. Therefore, volume expanders such as crystalloids may be utilized during a transfusion to maintain blood pressure and tissue perfusion. Due to the surplus of oxygen delivery in a normal physiological state, the body can maintain tissue oxygenation even when hemoglobin levels are below normal. Evidence shows that different hemoglobin thresholds are needed for transfusions to maintain adequate oxygen delivery to patients. However, it is noteworthy that these transfusion guidelines lack applicability in cases of acute blood loss.
Hemoglobin is a measurement of the concentration of hemoglobin molecules in the blood, and this level is not constant but rather can fluctuate. Therefore, in cases of acute blood loss, the hemoglobin concentration will remain unchanged. Patients who are mildly or moderately ill can receive volume expansion by using crystalloid solutions. Administering substantial volumes of crystalloid solutions to resuscitate severely injured patients carries the risk of dilutional coagulopathy.
Medical and military experience has shown that trauma patients benefit from receiving fresh whole blood, leading to the practice of transfusing PRBCs, platelets, and FFP in a 1:1:1 ratio. Controversy persists concerning the optimal ratio of these 3 components, with no compelling evidence indicating inferiority in lower ratios of platelets and FFP. Proponents of the 1:1:1 ratio highlight the potential benefits, such as preventing the excessive use of crystalloid solutions. This can help avoid dilutional coagulopathy, tissue swelling, delayed wound healing, extended reliance on ventilator support, and prolonged hospitalizations. Warming the blood helps prevent hypothermia, whereas the use of cryoprecipitate, fibrinogen concentrate, and recombinant factor VIIIa produces variable outcomes.
Studies have shown that severe trauma suppresses fibrinolysis. Compelling evidence from military studies suggests that tranexamic acid (TXA) can mitigate coagulopathy and improve survival rates among patients with combat injuries. Additional evidence supports the use of TXA in reducing mortality in the treatment of civilian trauma. TXA functions by blocking fibrinolysis or the breakdown of clots and is most effective when administered within 3 hours of the trauma. Administering TXA beyond 3 hours after the trauma leads to less favorable outcomes. As a result, many MTPs now incorporate TXA into their procedures.[12]
Initially, patients are given O-negative blood until crossmatched PRBCs become accessible. For the initial phase, it is advisable to have universal thawed plasma, typically AB plasma, readily available. Type A plasma with low titers of anti-B can also be a suitable choice. Patients should promptly receive group-specific plasma when their blood typing is completed.
Continuous monitoring throughout the resuscitation process is imperative. Protocols should include steps for assessing coagulopathy and overseeing the treatment of acid-base disorders, hypothermia, and electrolyte abnormalities. The typical practice is to assess the following parameters after approximately 5 units of PRBCs have been administered:
- Complete blood count (CBC) with platelet count
- Prothrombin time (PT)
- Activated partial thromboplastin time (aPTT)
- Fibrinogen concentration
Ideal monitoring involves measuring pH, blood gases, electrolytes, and metabolites such as glucose, and lactate every 20 to 30 minutes. TEG assesses platelet function, clot strength, and fibrinolysis. Therefore, when TEG is accessible, the results obtained from the test can inform decisions regarding the timing of additional plasma, cryoprecipitate, platelets, or antifibrinolytics, depending on the specific TEG profile.
The resuscitation objectives in the context of massive transfusion include:
- A mean arterial pressure (MAP) within the range of 60 to 65 mm Hg
- Hemoglobin level between 7 and 9 g/dL
- International normalized ratio (INR) below 1.5
- Fibrinogen levels within the range of 1.5 to 2 g/L
- Platelet counts above 50,000 μL
- pH between 7.35 and 7.45
- Core temperature above 35 °C
Complications
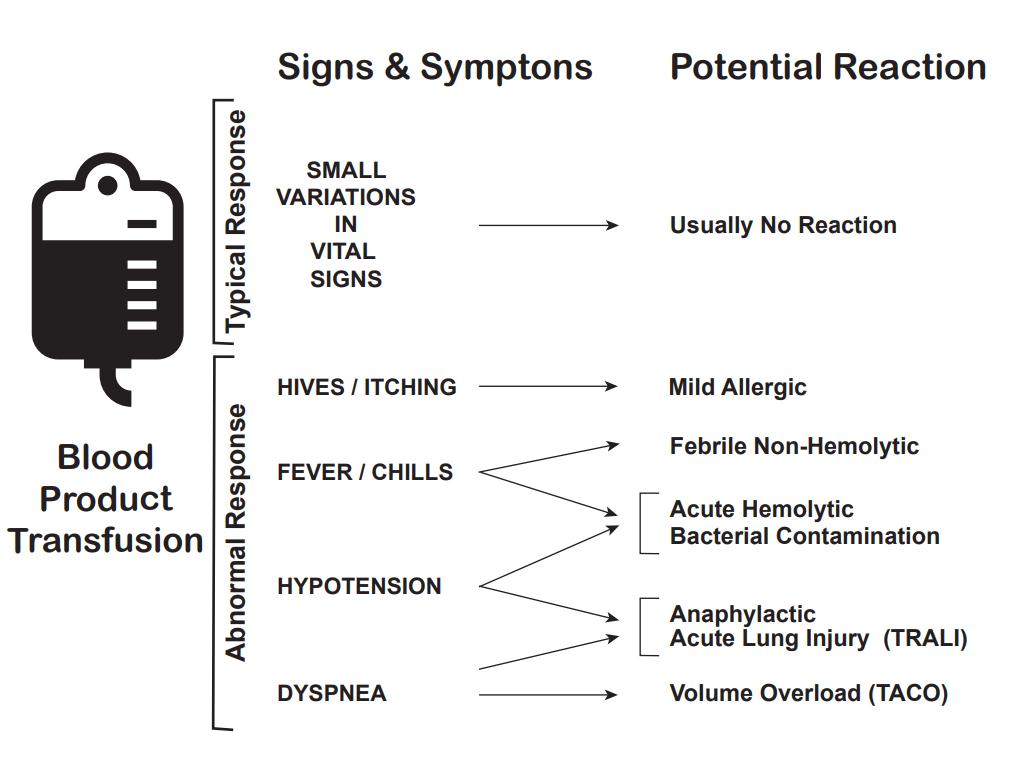
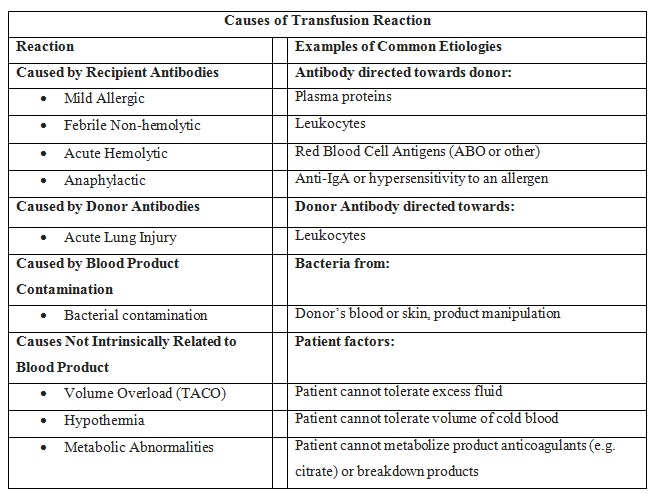
In addition to the mortality associated with the patient's injury or illness, massive transfusions have numerous potential complications (see Images. Transfusion Reaction Signs and Symptoms and Transfusion Reaction Causes). Vigilant monitoring of acid-base status, electrolytes, body temperature, volume status, tissue oxygenation, and coagulation parameters is essential. Non-fatal complications are observed in over 50% of patients when transfusing more than 5 units of blood products.[13][14][15]
Coagulopathy
Coagulopathy is a frequent occurrence attributed to the underlying condition and the transfusion. Coagulopathy arises from clotting factor consumption and activation due to tissue trauma, reduced clotting factor activity due to dilution, prolonged shock, hypoxia-induced acidosis, and hypothermia.[9] The administration of PRBCs and crystalloid infusions contributes to the dilution of clotting factors. To counter dilutional coagulopathy, the provision of plasma, fibrinogen, and platelets is essential. Due to periods of hypoperfusion, patients necessitating a massive transfusion frequently exhibit acidosis before the commencement of transfusion. Once acidosis is established, it exacerbates coagulation dysfunction by impeding the assembly of coagulation factors. There is a direct correlation between declining pH levels and decreased activity of coagulation cascade components. This leads to delayed and less robust fibrin clot formation.[16]
Metabolic Abnormalities
Sodium citrate and citric acid can result in metabolic alkalosis and hypocalcemia when added to blood products during storage to prevent coagulation. The metabolization of citrate to bicarbonate generates 23 mEq of bicarbonate per unit of blood. Metabolic alkalosis results if the kidneys are unable to excrete the excess bicarbonate. When citrate binds to ionized calcium, it can rapidly cause hypocalcemia, whereas calcium bound to albumin is unaffected. Severe hypocalcemia can lead to paresthesias and cardiac dysrhythmias. Regular monitoring of blood pressure and the corrected QT interval is crucial to prevent complications. The majority of patients do not require calcium supplementation unless they develop symptoms of hypocalcemia.
Furthermore, alkalosis can lead to hypokalemia as hydrogen ions exit cells to offset the alkalosis via an H+/K+ transporter. Citrate is present in FFP and platelets.[17] Administration exacerbates the hypocalcemia, resulting in more pronounced coagulopathy, necessitating additional transfusions, extended stays in the ICU, prolonged ventilator use, and heightened mortality rates. The administration of calcium did not demonstrate any benefit in mitigating these effects.
Notably, the potassium levels in the blood may increase during extended storage periods, thereby leading to hyperkalemia, which could potentially become a complication. In addition, hyperkalemia is also likely to occur when blood products are infused rapidly through central access.[5] Blood products with elevated extracellular potassium levels can potentially induce arrhythmias if directly infused into the heart. The primary issues arise from high-volume transfusions or blood exchange procedures involving heart-lung machines, extracorporeal membrane oxygenation (ECMO) devices, and apheresis machines that use older, cold-stored blood as priming solutions. Using younger PRBCs or washed RBCs can be beneficial to address this concern.
Hypothermia
Many patients with acute blood loss are susceptible to hypothermia, which can lead to coagulopathy. Lower ambient temperatures and decreased blood volume can further predispose these patients to hypothermia. Hypothermia reduces the efficacy of the coagulation cascade by reducing the enzymatic activity of coagulation proteins and platelet plug formation. Coagulation effects commence at 34 °C, and at 30 °C, there is an approximately 50% reduction in platelet activation. When stored at 4 °C, the rapid infusion of cold blood can induce a drop in core body temperatures. Many rapid infusers are equipped with warmers to mitigate the risk of hypothermia during massive transfusion.
Transfusion-Related Acute Lung Injury (TRALI)
Massive transfusion can cause TRALI [18][19] —a rapid-onset lung injury leading to non-cardiogenic pulmonary edema. TRALI type I manifests in patients without additional risk factors for acute respiratory distress syndrome (ARDS). However, TRALI type II occurs in patients with ARDS risk factors or those who already have ARDS but experience acute decompensation due to transfusion. Approximately 85% of cases result from the infusion of antibodies targeting human leukocyte antigen (HLA) or antineutrophil antibodies present in the donor blood products. The remaining 15% of cases likely originate from biological response modifiers, for instance, soluble CD40 ligand, within the stored blood product.
TRALI initiates when the recipient's neutrophils become primed to activate due to the patient's underlying condition. These primed neutrophils subsequently become activated by the antibodies targeting HLA or the biological response modifiers. Once activated, these neutrophils release cytokines, reactive oxygen species, oxidases, and proteases that damage the pulmonary capillary endothelium. The incidence of TRALI rises in direct proportion to the quantity of blood products administered.
Transfusion-Associated Circulatory Overload (TACO)
TACO refers to respiratory distress and pulmonary edema resulting from excess volume or circulatory overload. Unlike TRALI, TACO presents with visible neck vein distention, an S3 heart sound, and hypertension. This condition also responds well to diuresis and exhibits a pulmonary artery occlusion pressure exceeding 18 mm Hg. TACO develops when there is an overly aggressive transfusion or the administration of an excessive volume within a short time frame.[20] Both TRALI and TACO typically occur within 6 hours of a transfusion. Patients with TRALI clinically resemble those with ARDS, presenting with a PaO2/FiO2 ratio of less than 300 mm Hg but greater than 200 mm Hg, bilateral infiltrates on chest x-ray, and no signs of systolic heart failure. If symptoms occur in the 12 hours before the transfusion or more than 6 hours after a transfusion, then ARDS is the appropriate diagnosis.
Additional Complications
Acute hemolytic transfusion reaction: This reaction is characterized by symptoms such as fever, chills, flank pain, and oozing from IV sites, resulting from acute intravascular hemolysis of transfused red blood cells. The leading cause is typically ABO incompatibility or a reaction to other RBC antigens.
Transfusion-associated sepsis: This sepsis occurs when a patient is transfused with blood containing a microorganism, causing symptoms such as fever, chills, and hypotension.
Anaphylactic transfusion reaction: This is a severe allergic reaction characterized by angioedema, wheezing, and hypotension.
Allergic transfusion reaction: This allergic reaction commonly arises from antigen-antibody interactions between the patient and the transfused product, and it typically presents with symptoms limited to hives and itching. If no additional symptoms are observed, the transfusion can proceed.
Febrile non-hemolytic transfusion reaction (FNHTR): FNHTR occurs due to the release of cytokines from white blood cells in the transfused product, and fever is the sole symptom in this type of reaction.
Hypotensive transfusion reaction: These reactions are characterized by a significant drop in systolic blood pressure, typically 30 mm Hg or more. These reactions are most frequently associated with platelet transfusions. The underlying mechanism is likely related to vasoactive kinins. Patients taking angiotensin receptor inhibitors are at an elevated risk for hypotensive transfusion reactions.
At each stage of the transfusion process, a designated healthcare team member is responsible for confirming that the blood being administered to the patient matches the correct blood type. FNHTRs occur in 10% to 15% of patients who receive blood, and the clinical judgment of a healthcare professional guides the decision on whether to continue or halt the transfusion. If an adverse reaction occurs, it is essential to promptly halt the transfusion and inform the patient's healthcare provider. The healthcare team should ensure that the blood and all associated tubing are promptly returned to the laboratory. During blood transfusions, it is crucial to exercise caution as complications can be fatal.[21][22]
Clinical Significance
Hemorrhage is the second leading cause of death among trauma patients within the initial hour of their arrival at a trauma center. Massive transfusion is an essential life-saving intervention. Implementing MTPs reduced the percentage of hemorrhage-related deaths in a center from 38% to 25%.[23] Administering platelets and FFP at an earlier stage in the process decreases mortality.
Obstetrics, gastroenterology, the trauma bay, and the operating room frequently witness the activation of MTPs. Although the etiology of bleeding may vary in each case, the same principles of massive transfusion are applicable. Massive transfusions can lead to severe complications. Establishing protocols for initiating a massive transfusion and intratransfusion monitoring improves patient outcomes, reduces blood product consumption, and minimizes waste.[24][25][26]
Enhancing Healthcare Team Outcomes
Massive transfusions are necessary to treat massive hemorrhage. Interprofessional pre-planning is essential due to the unpredictable nature of such situations, the substantial personnel involvement, and the significant volume of blood products required. Implementing an MTP necessitates input from multiple teams, including trauma, surgical, anesthesia, and emergency medicine, as well as the blood bank. Clinicians must be adept at identifying the appropriate time to commence an MTP. Conducting regularly scheduled drills is pivotal for ensuring preparedness.
The availability of O-negative blood and thawed plasma in the blood bank facilitates the immediate infusion of blood products instead of crystalloid solutions. Algorithms must be established to continuously monitor acid-base status, coagulation factors, electrolytes, and body temperature. If TEG is accessible, it can be a valuable resource for determining the necessary blood products.
The potential for complications associated with massive blood transfusions is significantly high. Studies indicate that blood transfusions of more than 20 to 30 units do not enhance patient survival and are linked to a higher incidence of adverse events.[27][28] Familiarity with the facility's MTP, understanding when to activate it, and being aware of potential complications to monitor can significantly decrease morbidity and mortality rates while also reducing blood product utilization and waste.