[1]
Williamson JM, Thorn CC, Spalding D, Williamson RC. Pancreatic and peripancreatic somatostatinomas. Annals of the Royal College of Surgeons of England. 2011 Jul:93(5):356-60. doi: 10.1308/003588411X582681. Epub
[PubMed PMID: 21943457]
[2]
de Wilde RF, Edil BH, Hruban RH, Maitra A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nature reviews. Gastroenterology & hepatology. 2012 Feb 7:9(4):199-208. doi: 10.1038/nrgastro.2012.9. Epub 2012 Feb 7
[PubMed PMID: 22310917]
[3]
Ganda OP, Weir GC, Soeldner JS, Legg MA, Chick WL, Patel YC, Ebeid AM, Gabbay KH, Reichlin S. "Somatostatinoma": a somatostatin-containing tumor of the endocrine pancreas. The New England journal of medicine. 1977 Apr 28:296(17):963-7
[PubMed PMID: 321960]
[4]
Mansour JC, Chen H. Pancreatic endocrine tumors. The Journal of surgical research. 2004 Jul:120(1):139-61
[PubMed PMID: 15172200]
[5]
Mozell E, Stenzel P, Woltering EA, Rösch J, O'Dorisio TM. Functional endocrine tumors of the pancreas: clinical presentation, diagnosis, and treatment. Current problems in surgery. 1990 Jun:27(6):301-86
[PubMed PMID: 1973365]
[6]
Nesi G, Marcucci T, Rubio CA, Brandi ML, Tonelli F. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. Journal of gastroenterology and hepatology. 2008 Apr:23(4):521-6
[PubMed PMID: 17645474]
Level 3 (low-level) evidence
[7]
Vinik AI, Strodel WE, Eckhauser FE, Moattari AR, Lloyd R. Somatostatinomas, PPomas, neurotensinomas. Seminars in oncology. 1987 Sep:14(3):263-81
[PubMed PMID: 2820062]
[8]
O'Brien TD, Chejfec G, Prinz RA. Clinical features of duodenal somatostatinomas. Surgery. 1993 Dec:114(6):1144-7
[PubMed PMID: 8256221]
[9]
Soga J, Yakuwa Y. Somatostatinoma/inhibitory syndrome: a statistical evaluation of 173 reported cases as compared to other pancreatic endocrinomas. Journal of experimental & clinical cancer research : CR. 1999 Mar:18(1):13-22
[PubMed PMID: 10374671]
Level 3 (low-level) evidence
[11]
Tanaka S, Yamasaki S, Matsushita H, Ozawa Y, Kurosaki A, Takeuchi K, Hoshihara Y, Doi T, Watanabe G, Kawaminami K. Duodenal somatostatinoma: a case report and review of 31 cases with special reference to the relationship between tumor size and metastasis. Pathology international. 2000 Feb:50(2):146-52
[PubMed PMID: 10792774]
Level 3 (low-level) evidence
[12]
Mao C, Shah A, Hanson DJ, Howard JM. Von Recklinghausen's disease associated with duodenal somatostatinoma: contrast of duodenal versus pancreatic somatostatinomas. Journal of surgical oncology. 1995 May:59(1):67-73
[PubMed PMID: 7745981]
[13]
Öberg K, Knigge U, Kwekkeboom D, Perren A, ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2012 Oct:23 Suppl 7():vii124-30
[PubMed PMID: 22997445]
Level 1 (high-level) evidence
[14]
Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocrine-related cancer. 2011 Oct:18 Suppl 1():S1-16. doi: 10.1530/ERC-11-0013. Epub 2011 Oct 17
[PubMed PMID: 22005112]
[15]
Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best practice & research. Clinical gastroenterology. 2012 Dec:26(6):737-53. doi: 10.1016/j.bpg.2012.12.003. Epub
[PubMed PMID: 23582916]
Level 3 (low-level) evidence
[16]
Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, Meyer T, Newell-Price J, Poston G, Reed N, Rockall A, Steward W, Thakker RV, Toubanakis C, Valle J, Verbeke C, Grossman AB, UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012 Jan:61(1):6-32. doi: 10.1136/gutjnl-2011-300831. Epub 2011 Nov 3
[PubMed PMID: 22052063]
[17]
O'Grady HL, Conlon KC. Pancreatic neuroendocrine tumours. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2008 Mar:34(3):324-32
[PubMed PMID: 17967523]
[18]
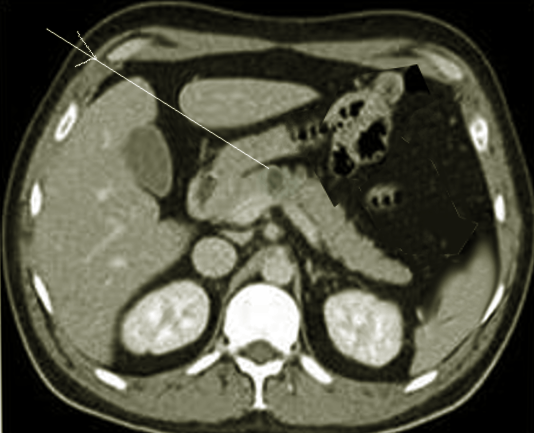
Tjon A Tham RT, Jansen JB, Falke TH, Lamers CB. Imaging features of somatostatinoma: MR, CT, US, and angiography. Journal of computer assisted tomography. 1994 May-Jun:18(3):427-31
[PubMed PMID: 8188911]
[19]
Singh S, Moody L, Chan DL, Metz DC, Strosberg J, Asmis T, Bailey DL, Bergsland E, Brendtro K, Carroll R, Cleary S, Kim M, Kong G, Law C, Lawrence B, McEwan A, McGregor C, Michael M, Pasieka J, Pavlakis N, Pommier R, Soulen M, Wyld D, Segelov E, Commonwealth Neuroendocrine Tumour Collaboration (CommNETS) Follow-up Working Group. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA oncology. 2018 Nov 1:4(11):1597-1604. doi: 10.1001/jamaoncol.2018.2428. Epub
[PubMed PMID: 30054622]
[20]
Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC, North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013 May:42(4):557-77. doi: 10.1097/MPA.0b013e31828e34a4. Epub
[PubMed PMID: 23591432]
Level 3 (low-level) evidence
[21]
Zakaria A, Hammad N, Vakhariya C, Raphael M. Somatostatinoma Presented as Double-Duct Sign. Case reports in gastrointestinal medicine. 2019:2019():9506405. doi: 10.1155/2019/9506405. Epub 2019 May 9
[PubMed PMID: 31210994]
Level 3 (low-level) evidence
[22]
Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2010 Aug:12(6):427-33. doi: 10.1111/j.1477-2574.2010.00198.x. Epub
[PubMed PMID: 20662794]
[23]
Berber E, Flesher N, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World journal of surgery. 2002 Aug:26(8):985-90
[PubMed PMID: 12016479]
[24]
Anene C, Thompson JS, Saigh J, Badakhsh S, Ecklund RE. Somatostatinoma: atypical presentation of a rare pancreatic tumor. The American journal of gastroenterology. 1995 May:90(5):819-21
[PubMed PMID: 7733095]
[25]
Doherty GM. Rare endocrine tumours of the GI tract. Best practice & research. Clinical gastroenterology. 2005 Oct:19(5):807-17
[PubMed PMID: 16253902]
[26]
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. The New England journal of medicine. 2011 Feb 10:364(6):501-13. doi: 10.1056/NEJMoa1003825. Epub
[PubMed PMID: 21306237]
[27]
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K, RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. The New England journal of medicine. 2011 Feb 10:364(6):514-23. doi: 10.1056/NEJMoa1009290. Epub
[PubMed PMID: 21306238]
[28]
Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. The New England journal of medicine. 1992 Feb 20:326(8):519-23
[PubMed PMID: 1310159]
[29]
Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011 Jan 15:117(2):268-75. doi: 10.1002/cncr.25425. Epub 2010 Sep 7
[PubMed PMID: 20824724]
[30]
Ramanathan RK, Cnaan A, Hahn RG, Carbone PP, Haller DG. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Annals of oncology : official journal of the European Society for Medical Oncology. 2001 Aug:12(8):1139-43
[PubMed PMID: 11583197]
[31]
Kulke MH, Wu B, Ryan DP, Enzinger PC, Zhu AX, Clark JW, Earle CC, Michelini A, Fuchs CS. A phase II trial of irinotecan and cisplatin in patients with metastatic neuroendocrine tumors. Digestive diseases and sciences. 2006 Jun:51(6):1033-8
[PubMed PMID: 16865563]
[32]
Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, Pacak K, Marx SJ, Kebebew E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016 Feb 20:34(6):588-96. doi: 10.1200/JCO.2015.64.0987. Epub 2015 Dec 28
[PubMed PMID: 26712231]
[33]
Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, Xu J, Soares K, Weiss MJ, Zheng L, Wolfgang CL, Cives M, Wong J, Wang W, Sun J, Shao C, Wang W, Tan H, Li J, Ni Q, Shen L, Chen M, He J, Chen J, Yu X. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Jan 20:35(3):274-280. doi: 10.1200/JCO.2016.67.8193. Epub 2016 Sep 30
[PubMed PMID: 27646952]
[34]
Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B, all other Frascati Consensus Conference participants, European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Archiv : an international journal of pathology. 2006 Oct:449(4):395-401
[PubMed PMID: 16967267]
Level 3 (low-level) evidence
[35]
Madeira I, Terris B, Voss M, Denys A, Sauvanet A, Flejou JF, Vilgrain V, Belghiti J, Bernades P, Ruszniewski P. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998 Sep:43(3):422-7
[PubMed PMID: 9863490]
[36]
House MG, Yeo CJ, Schulick RD. Periampullary pancreatic somatostatinoma. Annals of surgical oncology. 2002 Nov:9(9):869-74
[PubMed PMID: 12417508]
[37]
Li X, Gou S, Liu Z, Ye Z, Wang C. Assessment of the American Joint Commission on Cancer 8th Edition Staging System for Patients with Pancreatic Neuroendocrine Tumors: A Surveillance, Epidemiology, and End Results analysis. Cancer medicine. 2018 Mar:7(3):626-634. doi: 10.1002/cam4.1336. Epub 2018 Jan 29
[PubMed PMID: 29380547]
[38]
Skoura E, Michopoulou S, Mohmaduvesh M, Panagiotidis E, Al Harbi M, Toumpanakis C, Almukhailed O, Kayani I, Syed R, Navalkissoor S, Ell PJ, Caplin ME, Bomanji J. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016 Jan:57(1):34-40. doi: 10.2967/jnumed.115.166017. Epub 2015 Oct 15
[PubMed PMID: 26471695]
[39]
Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, Nieveen van Dijkum EJM, Pape UF, Pascher A, Ramage J, Reed N, Ruszniewski P, Scoazec JY, Toumpanakis C, Kianmanesh R, Falconi M, Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2017:105(3):255-265. doi: 10.1159/000464292. Epub 2017 Feb 25
[PubMed PMID: 28237989]
Level 3 (low-level) evidence
[40]
Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? Journal of the American College of Surgeons. 2000 Apr:190(4):432-45
[PubMed PMID: 10757381]