Introduction
Cerebrospinal fluid (CSF) has essential biochemical and mechanical functions. CSF is produced at a rate of around 500 ml/day; there are estimates that there is approximately 125 mL to 150 mL of CSF in the body at any given time. Depending on the rate of production and absorption (which varies individually), the supply of CSF can be replaced about every 7.5 hours. Most of this fluid is produced in the ventricles of the brain by the choroid plexus—however, the ependymal cells, which line the ventricles, produce a smaller portion. The choroid plexus arises from the walls of the lateral ventricles and the roofs of the third and fourth ventricles.
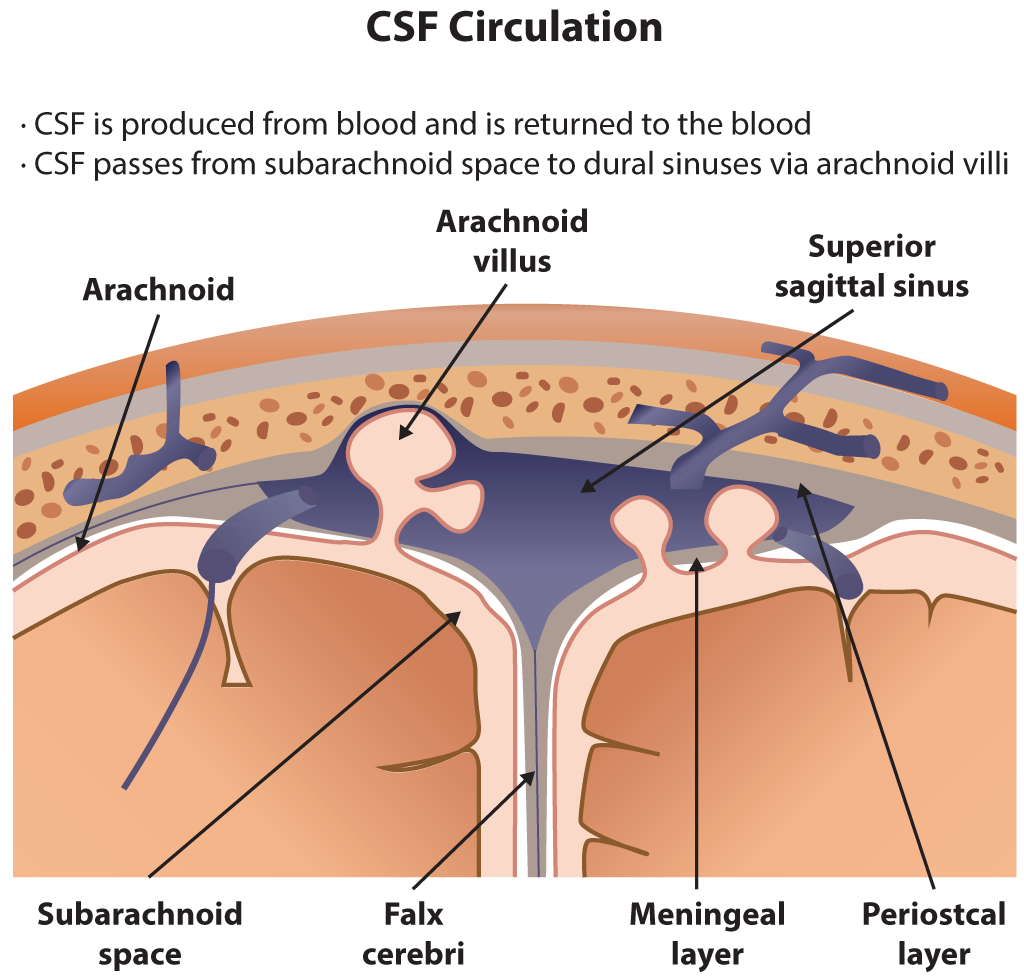
After production, CSF circulates through the ventricles, subarachnoid space, and parenchyma and is then ultimately reabsorbed into the venous circulation. It is reabsorbed into the blood through structures in the arachnoid mater called arachnoid villi (arachnoid granulations). Importantly, this fluid can be examined clinically through a lumbar puncture. With a lumbar puncture, physicians can look for abnormalities in the CSF, which can help create a differential diagnosis.[1][2]
Structure and Function
The composition of CSF is clear and colorless, with a density of between 1.003 to 1.008 g/cm.[3] On average, around 140 ml of CSF is divided between the ventricular system (approximately 35 ml), the spinal canal (~30-70 ml), and the subarachnoid spaces.[4] Compared to plasma, CSF has a lower glucose, protein, and potassium content with a higher chloride content.[3]
CSF appears to have several important functions. One of these functions is to provide a buoyant force to support the brain and spinal cord. The brain has a significant amount of mass (approximately 1500 gm) while at the same time being relatively malleable. However, since the CSF surrounds the brain, it dissipates much of the downward force that would normally act on the organ. This dissipation reduces the stress on the brain and allows it to maintain its shape. When surrounded by adequate CSF, the brain exerts significantly less tension on exiting nerve roots.[5] Another function of CSF that comes from its physical properties as fluid is to protect the brain from the damage that would result from a sudden movement of the skull. Any rapid acceleration/deceleration of the head has the potential to injure the delicate contents contained within. The CSF helps reduce the potential damage in such an event by acting as a cushion and a shock absorber.
Since there is continuous production of CSF, it also helps clear waste products from around the central nervous system and regulates intracranial pressure. This dynamic process can be controlled by either increasing or reducing production to match the central nervous system's needs at any given time. A similar regulatory mechanism can also reduce ischemia in the central nervous system (CNS) tissues. In times of ischemia, the volume of CSF can decrease and, therefore, decrease intracranial pressures. This decrease in intracranial pressure reduces the mechanical stress on blood vessels and may reduce ischemia.[6][7][8][9]
CSF also acts like a lymphatic system, allowing the movement of nutrients, metabolites, and toxins, and additionally has a role in neurodevelopment, chemical buffering, and homeostatic hormonal signaling mechanisms.[10][5][11]
Once formed, the CSF passes from the lateral ventricles through the foramen of Monro into the third ventricle. From the third ventricle, CSF then flows through the cerebral aqueduct of Sylvius into the fourth ventricle. From the fourth ventricle, the majority of the CSF exits the ventricular system through the paired lateral foramina of Luschka and the single medial foramen of Magendie into the various basilar cisterns and subarachnoid spaces, while a minority of CSF will continue into the central canal of the spinal cord. In the subarachnoid spaces, the CSF flows over the surface of the cerebral cortex. CSF is then drained back into the blood circulation via the arachnoid granulations into the cerebral venous sinus system.
Embryology
It is not currently known when the production of CSF first begins. However, the choroid plexus is visibly developing at around 32 days of development. The arachnoid villi also start to form at about 35 weeks, with continued development during the first 18 months of life.
Blood Supply and Lymphatics
The anterior choroidal artery (branch of the internal carotid artery) and the lateral posterior choroidal artery (branch of the posterior cerebral artery) are the main arterial blood supply to the choroid plexus of the lateral ventricles.[3] Arterial blood supply to the choroid plexus of the third and fourth ventricles is from the paired medial posterior choroidal arteries (branch of the posterior cerebral artery) and the posterior inferior cerebellar arteries, respectively.[3]
Surgical Considerations
Any neurosurgical intervention involving the ventricles, especially the external ventriculostomy, requires strict aseptic precautions as meningitis and ventriculitis can develop if infection infiltrates the CSF.
CSF Leak
Beta2-transferrin is only found in CSF, ocular fluids, and perilymph and is the desialated isoform of the iron-binding glycoprotein transferrin.[12] The presence of beta2-transferrin in a fluid sample can be used to confirm clinical suspicion of CSF leak.[12]
Ventricular Access
There are several common ventricular catheter insertion sites to access cerebrospinal fluid for CSF diversion.
- Kocher's point
- A localized entry point into the frontal horn of the lateral ventricle is anterior to the motor strip, commonly on the right side, to avoid the dominant hemisphere in most patients. Landmarks are 2-3 centimeters (cm) from the midline, which is approximately the mid-pupillary line. For insertion to be at least 1 cm anterior to the coronal suture, the target is approximately 11 cm posterior to the nasion. The ventricular catheter is then inserted perpendicular to the brain aiming toward the medial canthus of the ipsilateral eye in the medial plane and toward the external auditory meatus in the anterior-posterior plane.[13] CSF is typically obtained at a ventricular depth of <5 to 7 cm and may be at a depth of 3 to 4 cm in markedly dilated ventricles.
- There are multiple other commonly described points to surgically access the ventricular system, including Keen's point, Dandy's point, Frazier's point, and Paine's technique.[14][15][14][16]
Clinical Significance
Lumbar Puncture
An important clinical test that clinicians use to monitor CSF is the lumbar puncture (spinal tap). In this procedure, a long, hollow needle is typically inserted into the subarachnoid space of the lumbar spine just below where the spinal cord ends. The conus medullaris in most individuals ends at around the L2 level, while the thecal sac ends at around the S2 level. The needle can then be uncapped, and samples of CSF can be obtained. The first thing to note when doing a lumbar puncture is the opening CSF pressure. A high opening pressure can suggest various conditions, including hydrocephalus, intracranial mass, and idiopathic intracranial hypertension. When there is an elevated CSF pressure, there is a risk of damage to the brain by reducing the pressure too quickly.
For this reason, care is necessary to rule out intracranial space-occupying lesions and significantly associated mass-effect prior to performing this procedure. Intracranial space-occupying masses can be excluded on computed tomography (CT) imaging of the head or magnetic resonance imaging (MRI) of the brain. A low CSF pressure can also be clinically significant. This situation can occur in conditions that allow for CSF to leak into surrounding tissues. CSF can leak due to physical trauma, previous lumbar punctures, or without an obvious physical cause (spontaneous cerebrospinal fluid leak).
Several analyses are possible on the contents of CSF obtained from a lumbar puncture. Since CSF should be transparent, the color is worth noting. A cloudy appearance can suggest an infectious cause, and red or xanthochromic color can suggest the presence of blood. Cultures can be taken from this fluid to look for different infectious agents that can cause meningitis. Additionally, molecular analyses can help to look for protein abnormalities. These tests can help to diagnose a wide variety of diseases affecting the central nervous system.[17]
Hydrocephalus
Hydrocephalus is one important clinical disorder of cerebrospinal fluid. In this disorder, there is an abnormal accumulation of CSF within the ventricles. This typically results in elevated intracranial pressure. In the case of normal pressure hydrocephalus, there may be dilated ventricles in the absence of elevated intracranial pressure. Hydrocephalus is rarely due to CSF over-production and, in the majority of cases, is due to subnormal CSF reabsorption, which may occur in the case of choroid plexus papillomas or choroid plexus carcinomas. The two main functional subdivisions of hydrocephalus from subnormal CSF reabsorption are due to obstructive (non-communicating) hydrocephalus and non-obstructive (communicating) hydrocephalus.
Non-communicating hydrocephalus is due to an obstruction in CSF flow that is proximal to the arachnoid granulations. If there is a block in the cerebral aqueduct, for example, this may appear as enlargement of the lateral and third ventricle out of proportion to the fourth ventricle, commonly referred to as "triventricular hydrocephalus." Non-communicating hydrocephalus may also be due to intracranial space-occupying lesions. Communicating hydrocephalus is due to a defect in CSF reabsorption at the level of the arachnoid granulations.
Etiologies of hydrocephalus can be divided into congenital and acquired etiologies. Congenital etiologies include Chiari malformation, which may occur due to fourth ventricle outlet obstruction in Chiari I malformation and is commonly associated with myelomeningocele in Chiari type II. Other congenital etiologies for hydrocephalus include X-linked disorders, primary aqueductal stenosis, or Dandy-Walker malformation. Acquired etiologies of hydrocephalus include infectious, which is the most common cause of communicating hydrocephalus. Other acquired etiologies of hydrocephalus include post-hemorrhagic (following subarachnoid hemorrhage or intraventricular hemorrhage), neurosarcoidosis, post-operatively in pediatric patients who have undergone intracranial surgery or due to space-occupying intracranial lesions resulting in obstructive hydrocephalus. Some common locations of intracranial space-occupying lesions that may lead to obstructive hydrocephalus include a posterior fossa mass near the cerebral aqueduct, a sizeable suprasellar mass that may exert mass effect on the third ventricle, and a colloid cyst which can obstruct CSF flow at the foramen of Monro.
The treatment of hydrocephalus involves removing whatever obstructs CSF flow. In cases where there is recurrence or difficulty in ensuring a non-obstructed flow of CSF, a shunt can help drain excess fluid from the intracranial cavity. These shunts most often drain the CSF into the peritoneal cavity, but they can also be placed in other locations, such as the pleural cavity, gallbladder, and the right atrium of the heart.[18][19]
Aquaporin-4 is the principal water channel in the brain and is expressed in ependymal cells lining the ventricular system and perivascular astrocytes that are a part of the blood-brain barrier.[20] In recent years, studies have revealed a prominent role for aquaporin-4 in the production and circulation of CSF, suggesting it as a potential future therapeutic target for hydrocephalus.[21][22][23]
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension is characterized by an increased intracranial pressure (usually detected through lumbar puncture) with no discernable cause. In other words, it is unclear what is causing the increased CSF pressures. Patients often experience headaches, vision changes, pulsatile tinnitus, weakness, nausea, vomiting, etc. The diagnosis is generally made based on the history and physical exam. However, neuroimaging investigations and lumbar punctures are often necessary to rule out other potential causes.
Chiari Malformations
Chiari malformation is the name given to a group of deformities of the hindbrain (cerebellum, pons, and medulla oblongata).[24] There are two major types recognized:
- Chiari Type I Malformation (Hans Chiari)
- Occurs in 1 in 1,000 births; more common than type II malformations
- Genetic syndromes or, more commonly, idiopathic reduced volume of the posterior fossa lead to a displacement of the cerebellar tonsils into the spinal canal.
- Chiari Type II Malformation (Arnold-Chiari)
- Invariably results in the setting of myelomeningocele. Leakage of cerebrospinal fluid (CSF) to the myelomeningocele follows herniation of the hindbrain into the spinal canal, disturbing the cerebrospinal fluid's normal flow and potentially causing subarachnoid adhesions.