Continuing Education Activity
Continuous renal replacement therapy (CRRT) is a method of slower, continuous dialysis to allow solute and fluid homeostasis. There are different techniques of CRRT that are distinguished by their method of solute removal, each detailed below. The choice of CRRT is primarily based on provider preference instead of specific patient characteristics or outcome data. This activity details the principles, indications, and complications of CRRT, and it discusses when to consider initiating CRRT and when to discontinue it. It highlights the role of the inter-professional team in managing patients on CRRT.
Objectives:
- Describe the techniques of continuous renal replacement therapy.
- Identify the indications for continuous renal replacement therapy.
- Review the risks associated with continuous renal replacement therapy.
- Summarize the role of the interprofessional team in managing patients on continuous renal replacement therapy.
Introduction
Continuous renal replacement therapy (CRRT) is an available renal replacement method that includes intermittent hemodialysis and peritoneal dialysis. It is intended to be applied for 24 hours or longer through continuous, slower dialysis. CRRT is performed through pump-driven venovenous extracorporeal circuits and acts as renal support through blood purification to allow solute and fluid homeostasis.[1]
It requires appropriate vascular access, pumps to allow blood circulation, a permeable membrane, and varying solutions to allow fluid balance.[1] There are different techniques of CRRT that are distinguished by their method of solute removal, each detailed below. The choice of CRRT is primarily based on provider preference instead of specific patient characteristics or outcome data.[2]
Indications
The indications for renal replacement in the acute setting often include those usually indicated for dialysis in acute renal failure, such as volume overload, electrolyte disturbances including hyperkalemia, acidosis, and complications of uremia. The distinction comes in deciding on the method of renal replacement. The most common indication to choose CRRT is in hemodynamically unstable patients, and it is often employed in the intensive care unit. With a slower rate of fluid removal, CRRT theoretically causes less hypotension than IHD. Its second advantage for these patients is that they often require a large volume of fluid administration, including medications and parenteral nutrition, and CRRT can prevent an overloaded state. In fact, net fluid removal over 48 hours is greater than in an IHD session. Another reason to choose CRRT is for patients requiring dialysis who have an acute brain injury.[1]
The use of CRRT in these patients can help prevent worsening cerebral edema. IHD may worsen cerebral edema in two ways. The rapid fluid removal will lower mean arterial pressure and cause compensatory cerebral vasodilation. Rapid urea removal will cause fluid to shift from brain cells into the intracellular space [1]. Inflammatory mediators such as interleukin 6 (IL-6), IL-8, IL-1, and tumor necrosis factor-alpha may also be removed via convection.
The following is a list of indications to carry out CRRT in a patient:
- Volume overload[3][4][5]
- Metabolic acidosis[6][7][8]
- Electrolyte abnormalities[9][6]
- Hyperkalemia
- Hyponatremia
- Drug and toxin removal[10][11][12]
- Hyperphosphatemia
- Uremia[13]
- Encephalopathy
- Pericarditis
- Persistent/progressive acute kidney injury
Contraindications
The main contraindication for CRRT is the need to have treatment outcomes reached more rapidly than the CRRT treatment can accomplish. The following is a comprehensive list of contraindications to performing continuous renal replacement therapy:
- Advance directives indicating that the patient does not want dialysis
- Inability to establish vascular access
- Lack of expertise or the right equipment
- Irreversible liver failure when the patient is not a candidate for liver transplant
Equipment
CRRT is performed using a specialized machine comprised of filtration apparatus. The following is the list of medical products required to perform CRRT:
- Blood purification machine
- Dialysate
- Replacement fluid
- Filter
- Anticoagulation method
- Blood warmer
Personnel
Performing CRRT requires a collaborative team effort not only during the procedure but also prior to and after the procedure. The participation of experts in each domain, a specialist with expertise in critical nephrology, and a well-trained team of CRRT nurses may assist in priority establishment, implementation of quality control processes, and implementation of standardized policies.[14][15]
The involvement of specialists external to conventional intensive care staff with expertise in critically ill patients is not a new practice. The following is a list of external groups involved in the interprofessional management of patients on CRRT:
- Respiratory care practitioners
- Nutritional support team
- Clinical pharmacology
- Diagnostic and interventional radiology
- Cardiology
- Rehabilitation and physiotherapy[16]
Technique or Treatment
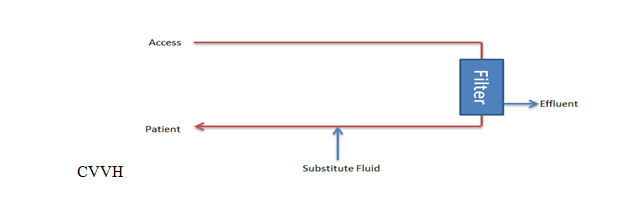
Continuous Venovenous Hemofiltration (CVVH): This method utilizes hydrostatic pressure across a semipermeable membrane for ultrafiltration using convection to filter solutes.[1][2] Higher and lower molecular weight solutes are transported with equal efficacy until the molecular radius of the solute exceeds the membrane pore size. A high ultrafiltration rate is required to allow sufficient solute clearance, so a substitute fluid is used to replace the filtered fluid. The substitute fluid can be introduced either before or after filtration. If the substitute fluid is introduced after the filter, the hemoconcentration occurring during filtration may increase the risk of sludging and occlusion with fibers. If introduced before, the blood will become diluted, and the risk of sludging will be reduced, but it may reduce effective solute clearance.[2]
Continuous Venovenous Hemodialysis (CVVHD): This method utilizes diffusion via a transmembrane concentration gradient across the membrane.[1] The concentration gradient is created using dialysate fluid. Solutes with small molecular weights, such as potassium, urea, and creatinine, are more easily transferred across the membrane than higher molecular weight solutes. The ultrafiltration rates a relatively low compared to CVVH.[2]
Continuous Venovenous Hemodiafiltration (CVVHDF): This combines both the convection and diffusion methods of filtration. In this method, dialysate is used in addition to the high rates of ultrafiltration and the use of replacement fluid. Small and middle-sized molecules are both efficiently removed.[1]
The composition of the dialysate or the substitution fluids can be tailored to achieve the desired plasma composition. This is mainly applied in electrolyte derangements and lactic acidosis, where a bicarbonate buffer may be used.
If CRRT is employed only for volume management without using a substitute fluid, it is known as slow continuous ultrafiltration.[1]
The preferred vascular access site is the right internal jugular vein due to a more direct pathway to the superior vena cava. An alternative to jugular access is the femoral vein. The subclavian vein is less preferred as it may result in stenosis of the vessel and may cause future issues if the patient requires an arteriovenous (AV) fistula. The ideal location of the catheter tip from an internal jugular approach is the junction of the superior vena cava and the right atrium. From a femoral approach, the ideal catheter tip location is close to or within the inferior vena cava. The positioning of the catheter needs to be able to sustain blood flow rates of around 200 to 300 mL/min. If the catheter is malpositioned, there is a higher chance of restricted blood flow, circuit flow interruptions, and filter clotting.[2]
End-stage renal disease (ESRD) patients with AV fistulas should not use these if CRRT is needed. Using their established AV fistula increases the risk of needle dislodgment and bleeding. Existing tunneled catheters dialysis may be used.
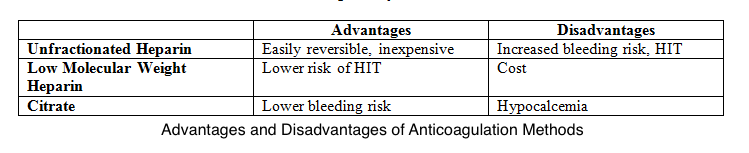
In CRRT, using an extracorporeal circuit results in a prothrombotic state. If membrane clotting occurs, it will decrease the surface area available for diffusion or convection. In this situation, an anticoagulant is required to prevent clotting. The anticoagulant used can either be systemic or regional. Both systemic and regional anticoagulation have advantages and disadvantages as shown in Table 1. The options for systemic anticoagulation include unfractionated heparin or low molecular weight heparin.[1] If a patient has heparin-induced thrombocytopenia (HIT), alternatives such as argatroban or hirudin may be used for systemic anticoagulation. Regional anticoagulation is achieved using citrate. Citrate acts by chelating calcium and thus inhibiting the clotting cascade.[1]
If using this method, serum ionized calcium levels should be monitored frequently. These patients will often require a calcium infusion with rate adjustments based on the ionized calcium level. One option if anticoagulation cannot be used is intermittent saline flushes. This is performed every 15 to 30 minutes and helps wash off the fibrin strands. However, filter half-lives are generally reduced.[1]
Table 1. Advantages and disadvantages of anticoagulation methods.
Complications
As with every procedure, CRRT has risks, and these should be communicated to the patient or family when considering initiation. Firstly, the risks associated with intravascular lines include hemorrhage, AV fistula, infection, or thrombosis. The risks of the therapy itself include electrolyte disturbances, clearance of trace elements or antibiotics, hypothermia, and hypotension.[17]
Although hypotension should occur less commonly than in IHD, hypotension may occur if the net ultrafiltration rate exceeds the intravascular filling rate. Monitoring of electrolytes and acid-base status should be done every 6 to 12 hours when starting CRRT. If remaining stable after the first 24 to 48 hours, the interval may be increased to 12 to 24 hours.[1] The exception, as discussed above, is when using citrate as regional anticoagulation because this requires frequent monitoring of ionized calcium levels.
The removal of medications during CRRT is variable, and so it is advised to check the dose of required drugs when on CRRT. This practice is critical when it comes to administering antibiotics, as the trough concentrations of these medications will determine their bacteriocidal or bacteriostatic effectiveness. Most commonly affected are water-soluble antimicrobials, aminoglycosides, and beta-lactam antibiotics.[17] Many patients who meet indications for CRRT will do so because of sepsis, meaning that appropriate antibiotic dosage is vital.
CRRT will result in amino acid, micronutrient, and water-soluble vitamin loss. Patients are also often in a substantial negative nitrogen balance. Appropriate caloric and protein intake with supplementation of water-soluble vitamins should be ensured.[2]
Finally, the risks associated with the extracorporeal circuit include hypersensitivity to the circuit, air embolization, and blood loss that occurs with the filter or circuit changes.[17]
Clinical Significance
When to Initiate
There are many factors to consider when deciding whether to initiate CRRT. Two main factors are the severity of the illness and the necessity of the procedure. The severity of the disease may be judged through the severity of the AKI and the observed trend. Further supporting factors would be the presence of electrolyte and acid-base disorders, evidence of fluid overload, and other significant organ dysfunction that requires renal support for promoting recovery. Without specific indications, the timing of initiation is up for debate.
Early initiation may allow for early correction of electrolyte abnormalities, volume status, and azotemia before significant disturbances can occur but must be weighed against complications. The trajectory of an AKI is difficult to predict, so isolating those patients likely to have a persistent AKI is not always reliable.[2] Factors that may aid in the assessment include the likelihood of the reversibility of the AKI, the presence of oliguria, and the nature and timing of the renal insult.
When to Discontinue
Every patient on CRRT should have daily monitoring for renal recovery. There are no standards for dialysis discontinuation. One way of monitoring renal recovery is by measuring urine output; increased urine output is an indicator of improving renal function. Once the intrinsic kidney function has improved enough that it is able to support the patients’ needs, CRRT may be discontinued.[18] In addition to renal function, other factors such as fluid overload, ongoing hemodynamic instability, or continued need for nephrotoxic medication or large volumes may need to be considered before stopping CRRT.
Enhancing Healthcare Team Outcomes
CRRT requires an interprofessional healthcare team approach to be provided safely and efficiently. It requires the collaboration of specialties, including critical care, nephrology, and neurology, on essential elements such as when to initiate a mode of clearance, solute and fluid removal targets, and anticoagulation strategies. All clinicians involved in the case, including nurse practitioners and physician assistants, must be contributors to care. Nursing is vital during CRRT as they have the most exposure to the vascular access site, the CRRT circuit itself, and the patient.
Nursing staff and patient care technicians should be aware of complications so that intervention can be initiated early, and the clinician team should be alerted promptly. In addition, pharmacists and nutritionists are vital to ensure proper medication doses and nutrition while on CRRT. Pharmacists will provide input on how CRRT may affect the patient's drug regimen and possible therapeutic outcome and needs to share their expertise with the rest of the team so any necessary adjustment to drug therapy can be put in place.
With this team approach and good education for each member, CRRT may be delivered as safely and effectively as possible.[19]
Nursing, Allied Health, and Interprofessional Team Interventions
The following are the roles provided by the interprofessional team:
- Collaboration between physician teams
- Define the primary goal of CRRT
- Ensure adequate access, machine, and anticoagulation to maintain high-functioning CRRT with minimal disruptions
- Ensure appropriate nutrition support
Nursing, Allied Health, and Interprofessional Team Monitoring
It is essential to continue monitoring patients getting CRRT in the following ways:
- Daily reassessment of CRRT prescription and response
- Close attention to the appropriateness of medication dosing
- Close lab and circuit monitoring for CRRT complications