Introduction
Apoptosis, first identified in the 1970s, was initially perceived as a process comparable to mitosis. Commonly referred to as programmed cell death, it plays an essential role in maintaining a balance with mitosis by regulating cell populations during development, preserving tissue homeostasis in adults, and contributing to various biological processes.[1] Over time, apoptosis was more clearly defined as a genetically programmed, ATP-dependent, enzyme-driven mechanism that eliminates cells deemed unnecessary or potentially harmful to the organism.[2]
Apoptosis results when the cytoskeleton is broken down by proteases and DNA is degraded by endonucleases through several pathways that are homeostatic or pathological. The process maintains homeostasis when cells compromise the organism's survival, but apoptosis does not occur at the appropriate rate or in the correct sequence when the process is no longer regulated.[3] Caspases mediate the process through the downstream effects of the upstream activation by intrinsic and extrinsic pathways, either working separately or simultaneously.[4]
Causes
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Causes
Current research suggests that apoptosis is one of the predominant cell death mechanisms, summarized below:
- Necroptosis: Occurs following activation of tumor necrosis factor-alpha (TNF-α), triggering several cell death receptors.[5]
- Pyroptosis: Mainly affects cell membrane integrity, engaging inflammasomes to activate caspases.[5][6]
- Apoptosis: Differentiated by the release of cytochrome c from the mitochondria, it is immunologically silent and non-lytic.[5][6]
- Ferroptosis: Characterized by iron-dependent phospholipid peroxides that accumulate in cell membranes, leading to non-apoptotic death.[7][8]
Given common mid-stream mediators, some researchers do not differentiate necroptosis from apoptosis as a separate mechanism. Instead, the simultaneous process is called PANoptosis, where pyroptosis, apoptosis, and necroptosis occur as programmed cell death.[9] In addition, autophagy refers to the process of digesting organelles or other parts of cells through lysosomal machinery, which can also lead to cell death.[10] Some parts of apoptosis are considered reversible, a phenomenon known as anastasis, particularly observed in cancer cell lines.[11]
Anatomical Pathology
Apoptosis is distinct due to the cascade of programmed cell death. Dying cells undergo shrinkage due to the disruption of the cell cytoskeleton, mainly caused by caspases. The cells become deeply eosinophilic, shrinking and distancing from their neighbors with the loss of cell-to-cell contact. The nucleus of the dying cell turns deeply basophilic. The hallmark of apoptosis is pyknosis, in which nuclear chromatin condenses to form 1 or more dark-staining masses against the nuclear envelope. Dissolution of the nuclear membrane occurs, and endonuclease slices the DNA into short, regularly spaced fragments (karyorrhexis).[3]
Subsequently, the condensed cytoplasm and nucleus break into fragments called apoptotic bodies that bud off from the cell. Macrophages then remove these apoptotic bodies in a process called efferocytosis. The cell membrane remains intact without inflammation, unlike necrosis, pyroptosis, or ferroptosis, where cell swelling and inflammation are common. Macrophages remove apoptotic cells quickly, with little or no inflammation occurring in the surrounding tissues. As such, the mechanism is considered immunologically silent.[12]
Clinical Pathology
Detecting apoptosis effectively requires the use of multiple assays, as its multi-stage complexity cannot be fully captured by a single method. Below is an overview of key assays organized by their specific detection targets.
Cytomorphological Changes
Apoptotic cells display distinct features such as chromatin condensation and membrane blebbing. Chromatin changes can be observed using DNA-binding dyes such as 4',6-diamidino-2-phenylindole (DAPI) or Hoechst, which emit brighter fluorescence in condensed nuclei under microscopic examination. Membrane blebbing, visible through phase-contrast microscopy in live cells, is linked to caspase-driven cleavage of proteins such as gelsolin and ROCK-1 kinase. Although fixed cells can be analyzed using caspase substrate markers, this approach may lead to false positives.[3]
DNA Fragmentation
A hallmark of late-stage apoptosis is the breakdown of DNA into 180 to 200 bp fragments. The TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay commonly labels 3'-OH DNA ends with fluorescent markers, allowing detection through microscopy or flow cytometry. Alternatively, DNA laddering through agarose gel electrophoresis offers a more affordable but less sensitive option.[13]
Caspase Activity, Cleaved Substrates, and Regulatory Proteins
Caspase activation, particularly caspases-3, -8, and -9, can be measured using fluorogenic substrates or fluorescent inhibitors. Mid-stage apoptosis is often confirmed by detecting poly ADP-ribose polymerase cleavage through Western blot analysis. In addition, the balance between pro- and anti-apoptotic proteins such as Bcl-2 and Bax can be assessed using immunoassays to evaluate regulatory mechanisms.[14]
Membrane Changes
Early apoptosis is characterized by phosphatidylserine externalization, which is detected using Annexin V staining. This staining is often combined with propidium iodide to distinguish apoptotic cells from necrotic ones. In later stages, loss of membrane integrity is monitored using dye exclusion assays.[15]
Whole-Mount Detection
Apoptosis in embryos or tissues can be detected using acidophilic dyes such as acridine orange, Nile blue sulfate, and neutral red. However, these dyes require validation due to nonspecific debris staining and have limitations such as toxicity and poor penetration. LysoTracker Red provides stable, three-dimensional imaging in thick tissues through confocal microscopy.[3]
Mitochondrial Assays
Mitochondrial assays for apoptosis detection focus on key changes, including mitochondrial membrane potential loss, which is detected using dyes such as JC-1, tetramethylrhodamine methyl ester, or tetramethylrhodamine ethyl ester.[3] Permeability transition pore opening is monitored with calcein fluorescence quenching. In addition, cytochrome c release is tracked through immunofluorescence or subcellular fractionation. These assays are often combined with caspase activity measurements for improved specificity.[16]
Mechanisms
Cell proliferation and cell death are balanced in all normal tissues of multicellular organisms. This normal cell death, vital for cell development and health, is called apoptosis and involves the following pathways. All the pathways involve the activation of caspases as the final step.
Intrinsic Pathway
The intrinsic pathway is activated when the cell experiences internal stress due to various factors such as DNA damage from x-ray or UV light exposure, chemotherapeutic agents, hypoxia, or the accumulation of misfolded proteins inside the cell, as observed in conditions such as Alzheimer's disease, Parkinson's disease, or Huntington disease. When the cell undergoes stress, cytochrome c leaks from the intermembrane space of mitochondria into the cytosol, which leads to the activation of caspase-9. The regulation of this pathway is governed by the Bcl-2 and TP53 genes.[17][18]
The Bcl-2 protein family includes both anti-apoptotic and pro-apoptotic members. Pro-apoptotic proteins detect death signals and trigger cell death, whereas anti-apoptotic proteins block this process. These evolutionarily conserved proteins may operate through varied mechanisms across species. Diverse cellular signals can alter their activity and location, creating a complex network that balances cell survival and death. Due to their key role in managing apoptosis from various triggers, Bcl-2 family proteins are vital for embryonic development and adult homeostasis.[19][20]
Extrinsic Pathway
The extrinsic pathway is triggered when the cell receives death signals from other cells. This pathway is receptor-linked, and the ligands from the other cells bind to these death receptors on the cell surface, activating apoptosis. This process involves the following cell surface receptors and their corresponding ligands, ultimately activating caspase-8, a key regulator in the cascade.
- TNF-α: TNF-α is a cytokine produced by macrophages and is the major extrinsic mediator of apoptosis. TNF-α binds to the receptor TNFR1, thereby activating caspases.
- Fas: T cells generate a surface receptor called Fas, which increases production during an infection. After a few days, the activated T lymphocytes release Fas ligands. When Fas binds to these ligands on the same or different cells, apoptosis is triggered through caspase activation. Fas receptor, a transmembrane protein of the TNF family, interacts with FasL to activate caspases. Apoptosis aids in the removal of the activated T lymphocytes when the infection has been cleared.
- Bcl-2 genes: These anti-apoptotic genes are located on chromosome 18 and produce the protein Bcl-2. Bcl-2 binds to and inhibits APAF-1, preventing the release of cytochrome c from the mitochondria. Cytochrome c is present between the inner and outer mitochondrial membranes. Cytochrome c release leads to its binding with APAF-1, activating procaspase-9.
- TP53 suppressor gene: This gene encodes a protein that regulates the cell cycle and promotes tumor suppression. Suppose DNA is damaged by ionizing radiation, chemotherapeutic agents, or hypoxia. In that case, TP53 arrests the cell in the G1 phase of the cell cycle, preventing the proliferation of cells with damaged DNA and facilitating DNA repair. Severe DNA damage prompts apoptosis by activating BAX apoptosis genes. BAX gene products inactivate the Bcl-2 anti-apoptosis gene.[21] The balance between pro-apoptotic and anti-apoptotic genes regulates the process (see Image. DNA Repair and Apoptosis).
- Cytotoxic CD8+ T-cell pathway: CD8+ T cells secrete perforins, creating holes in the target cells. Subsequently, CD8+ T cells secrete granzymes that enter the target cells through these holes and activate caspases.
- Caspases: Caspases are a group of protease-like enzymes. These enzymes exist in the cell in an inactive form and require proteolytic cleavage to become active. As described above, these enzymes are the primary effectors of apoptotic responses, activated by several regulators.[22]
- Initiator caspases include caspases 2, 8, 9, and 10. When activated, the initiator caspases activate the effector caspases.
- Effector caspases encompass caspases 3, 6, and 7. Active effector caspases cleave several proteins in the cell, leading to cell death and, ultimately, phagocytosis and removal of cellular debris.
- Of all the caspases, caspase-3 is the most frequently activated, which catalyzes the cleavage of major cellular proteins and condensation of chromatin. Caspase also activates DNAse enzymes, which causes DNA fragmentation followed by internucleosomal fragmentation.[23][24]
Execution Pathway
The execution phase, the final step of apoptosis, dismantles the cell through effector caspases, such as caspases 3, 6, and 7, which are activated by upstream caspases from either the extrinsic or intrinsic pathways. These enzymes degrade the nuclear envelope, block DNA repair, and fragment DNA into a distinct pattern through an activated nuclease, marking irreversible breakdown. The cell shrinks, alters its membrane to expose phagocytic signals, and forms debris-filled vesicles, which are cleared by phagocytes to prevent inflammation. Pro-apoptotic and anti-apoptotic proteins tightly regulate this orderly process.[25]
Other Players
Following initial cell death, several danger-associated molecular patterns and pathogen-associated molecular patterns are released from the eliminated cells, signaling additional inflammatory mediators depending on the type of cell death and if other mechanisms are involved. Consequently, whether apoptosis is completely immunologically silent is still debated.[5] Apoptosis proteins are believed to be inhibited in several pathological conditions, particularly cancer, where apoptosis is typically suppressed. These modulators are a family of anti-apoptotic proteins called inhibitors of apoptosis proteins.[26] Cathepsin D is believed to trigger apoptosis, especially during tissue remodeling.[27]
Clinicopathologic Correlations
Embryogenesis
During embryogenesis in the fetus, the formation of the digits involves the apoptosis of interdigital tissues. Similarly, several body cavities undergo apoptosis in utero.[28] For example, a male fetus loses Müllerian structures due to a Müllerian inhibitory factor synthesized by Sertoli cells.[29]
Menstrual Cycle
The sloughing of the inner lining of the uterus (the endometrium) after the withdrawal of estrogen and progesterone in the menstrual cycle is a physiological process of apoptosis.[29]
Immunological Regulation
- Virus-infected cells: Cytotoxic T cells kill the virus-infected cells by apoptosis.
- Cells with DNA damage: Cells whose DNA is damaged by radiation exposure or chemotherapeutic agents are arrested in the G1 phase of the cell cycle for repair by activating p53. P53 is a tumor suppressor gene. A p53 mutation inhibits apoptosis, leading to the survival of abnormal cells and the development of carcinomas.
- Autoreactive T cells: Autoreactive T cells in the thymus are eliminated through apoptosis.[30]
Apoptosis is required for the development and maintenance of a healthy immune system. When B and T lymphocytes are initially produced, they are tested to see whether they react against any of the body's self-components. Cells that react are killed by apoptosis. If these cells are not removed, self-reactive cells may be released into the body, which can attack tissues and cause autoimmune conditions. Apoptosis is required to turn off the immune system after the offending pathogen is cleared from the body, such as removing acute inflammatory cells, including neutrophils, from healing sites. Furthermore, the destruction of B and T lymphocytes by corticosteroids occurs through apoptosis.[31]
Removal of Misfolded Proteins
Apoptosis removes misfolded proteins, such as amyloids and proteins in prion-related diseases. As a result, several formations that may lead to neurodegenerative diseases are eliminated.
Clinical Significance
Tumorigenesis
A decrease in apoptosis results in higher cell survival rates, leading to the development of cancers. In follicular lymphoma, a translocation event relocates the Bcl-2 gene from chromosome 18 to chromosome 14, leading to excessive transcription and increased levels of Bcl-2. Elevated Bcl-2 gene levels inhibit APAF-1, consequently inactivating caspases and intrinsic apoptosis, leading to follicular lymphoma. Mutations or deletions of the p53 gene increase the risk of tumor formation by enabling cells with damaged DNA to divide uncontrollably.[31]
Factors such as exposure to chemicals, radiation, and viruses can damage the p53 gene. Individuals with Li-Fraumeni syndrome have only 1 functional copy of p53; therefore, they are more likely to develop a tumor in early adulthood. When DNA repair mechanisms fail to remove the damaged, translocated, or deleted DNA, cells begin to evade cell cycle checkpoints that lead to apoptosis (see Image. The Mechanism of Apoptosis).[32][33]
Autoimmune Diseases
A decrease in the apoptosis of self-reactive immune cells can lead to the development of autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and autoimmune lymphoproliferative syndrome.[34] Recently, the role of mitochondria in regulating cell death has been linked to the development of diabetes due to the destruction of β-cells.[35]
Neurodegenerative Diseases
Cell death has also been implicated in many neurodegenerative disorders. Necrosis and apoptosis occur in neurologic diseases such as acute ischemic syndrome. In chronic neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, and Huntington's disease, neuronal cell death predominantly occurs through apoptosis and has been implicated.[36]
Cardiovascular Diseases
Necrosis was long considered the sole cause of myocardial infarction. However, recent studies have shown that apoptosis also occurs mainly during the reperfusion phase after the acute infarction, leading to further myocardial damage. Staging atherosclerotic plaques and rupture is correlated to apoptosis, specifically the death of macrophages.[10][37]
Therapeutic Implications
Given the correlation of physiological and pathological processes, the identified players in intrinsic and extrinsic apoptosis are targets for immunotherapy.[37] Several cancer therapies inhibit Bcl-2, which is the most notorious example.[38] Given the multilevel effects of apoptotic pathways, no one specific therapeutic innovation has set the standard. Recently, a TUNEL assay was used to quantify apoptotic cell death, which is pertinent when staging pathological tissue samples.[11] Similarly, the applicability of antibody-specific inhibitors to malignant tumor antigens is perhaps the next level of treatment specificity.[9]
Media
(Click Image to Enlarge)
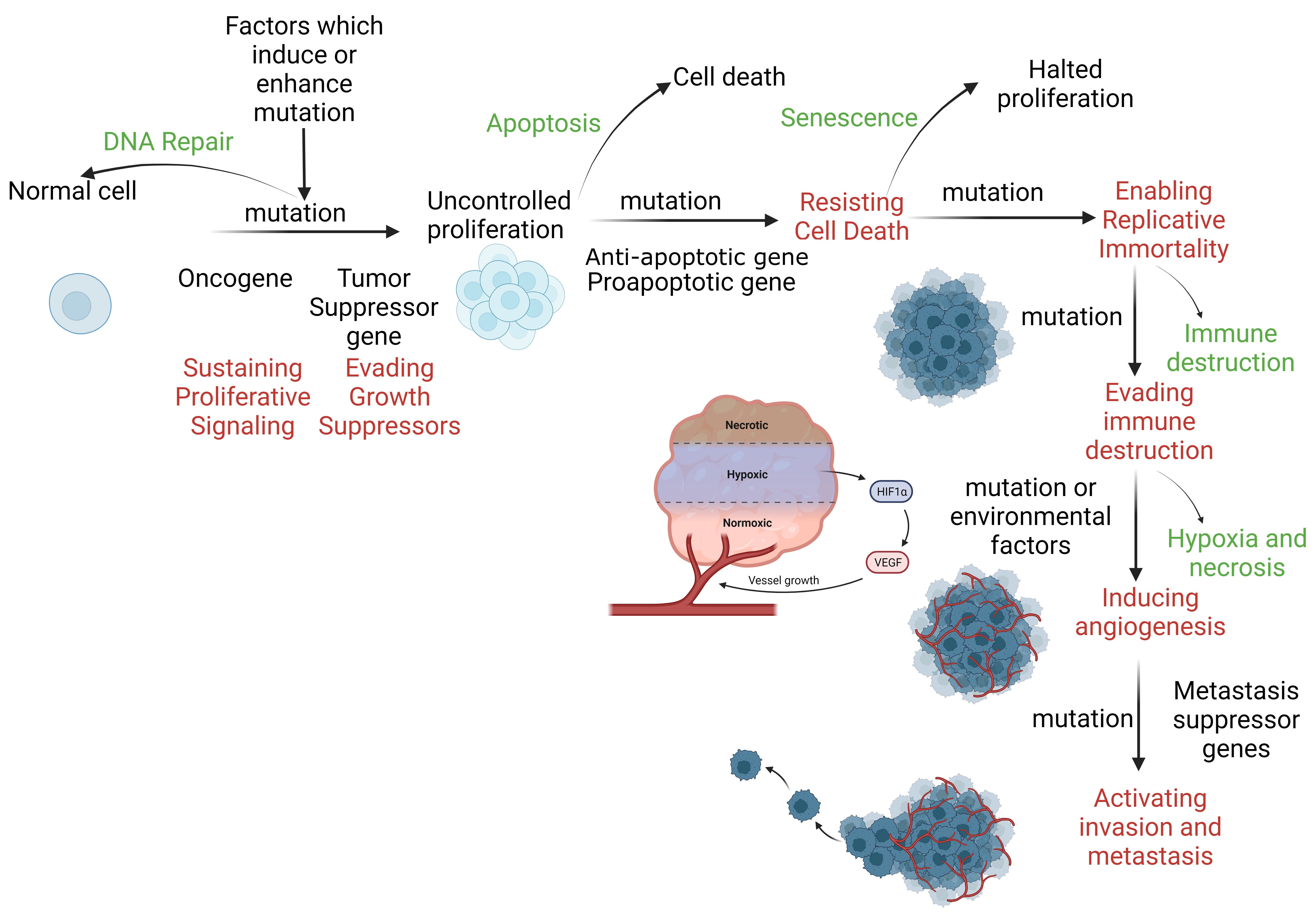
DNA Repair and Apoptosis. DNA repair restores a cell to its normal state, whereas apoptosis eliminates a cell without inflammation. Defective DNA repair and apoptosis allow the cell to initiate uncontrolled proliferation through promotion and subsequent progression, resulting in distant tissue invasion. HIF-1α, hypoxia-inducible factor 1 alpha; VEGF, vascular endothelial growth factor.
Contributed by S Abd El Fattah, MD
(Click Image to Enlarge)
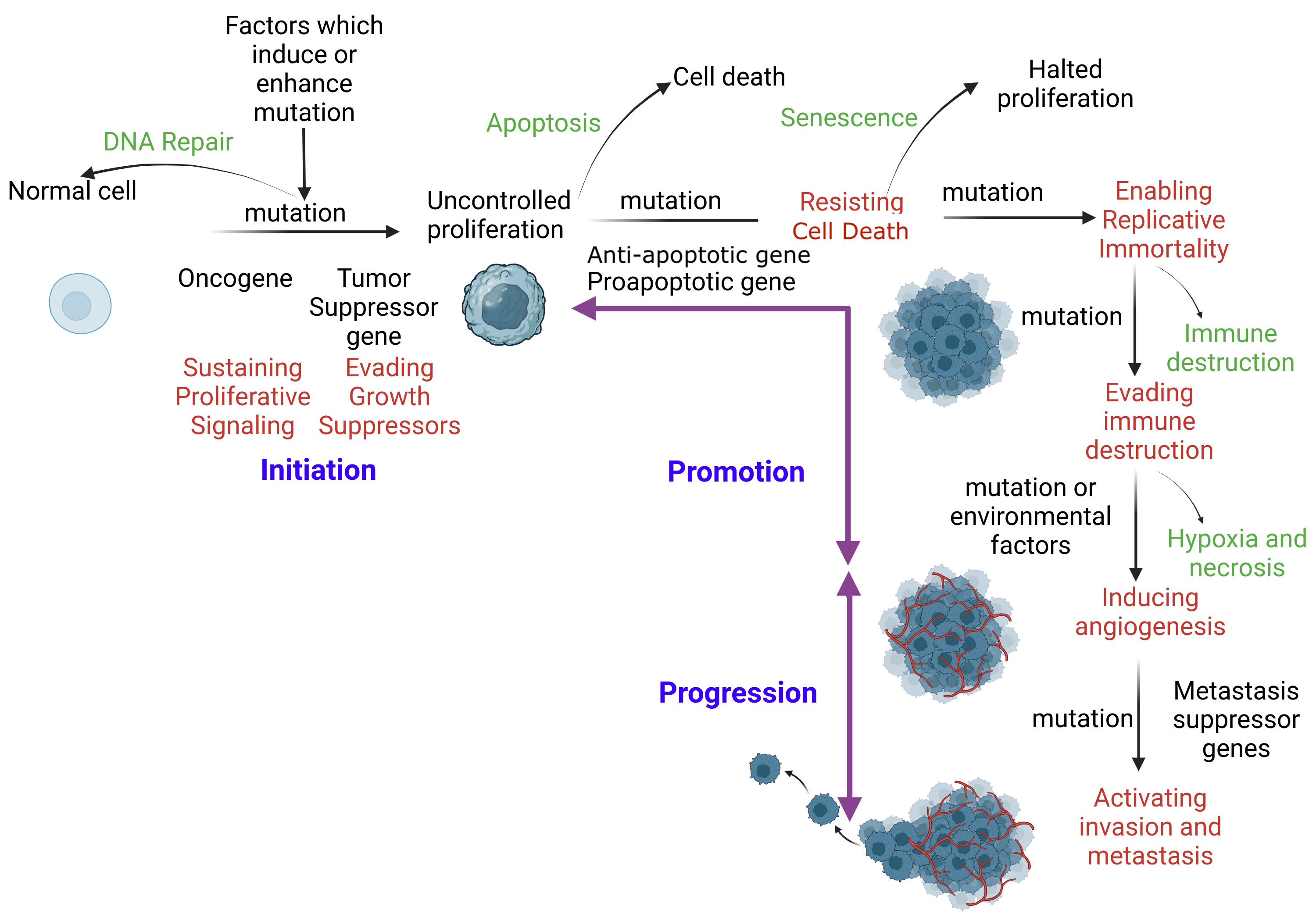
Mechanism of Apoptosis. When DNA repair fails, apoptosis ensues to eliminate the corrupt cell without inflammation. An initiated cell evades tumor suppressor genes and activates constitutional proliferation (auto-proliferation). Subsequently, the initiated cell is promoted through the acquisition of additional mutations. This mutation immortalizes the cell by skipping immune checkpoints. Additional mutations are acquired, facilitating distant tissue invasion.
SA Ibrahim, MBBCh, MSc, PhD
References
Park W, Wei S, Kim BS, Kim B, Bae SJ, Chae YC, Ryu D, Ha KT. Diversity and complexity of cell death: a historical review. Experimental & molecular medicine. 2023 Aug:55(8):1573-1594. doi: 10.1038/s12276-023-01078-x. Epub 2023 Aug 23 [PubMed PMID: 37612413]
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell death and differentiation. 2018 Mar:25(3):486-541. doi: 10.1038/s41418-017-0012-4. Epub 2018 Jan 23 [PubMed PMID: 29362479]
Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology. 2007 Jun:35(4):495-516 [PubMed PMID: 17562483]
Level 3 (low-level) evidenceKumar S, Dorstyn L, Lim Y. The role of caspases as executioners of apoptosis. Biochemical Society transactions. 2022 Feb 28:50(1):33-45. doi: 10.1042/BST20210751. Epub [PubMed PMID: 34940803]
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cellular & molecular immunology. 2021 May:18(5):1106-1121. doi: 10.1038/s41423-020-00630-3. Epub 2021 Mar 30 [PubMed PMID: 33785842]
Ketelut-Carneiro N, Fitzgerald KA. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. Journal of molecular biology. 2022 Feb 28:434(4):167378. doi: 10.1016/j.jmb.2021.167378. Epub 2021 Nov 25 [PubMed PMID: 34838807]
Samir P, Malireddi RKS, Kanneganti TD. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Frontiers in cellular and infection microbiology. 2020:10():238. doi: 10.3389/fcimb.2020.00238. Epub 2020 Jun 3 [PubMed PMID: 32582562]
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P. Ferroptosis: mechanisms and links with diseases. Signal transduction and targeted therapy. 2021 Feb 3:6(1):49. doi: 10.1038/s41392-020-00428-9. Epub 2021 Feb 3 [PubMed PMID: 33536413]
Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024 Jan 18:187(2):235-256. doi: 10.1016/j.cell.2023.11.044. Epub [PubMed PMID: 38242081]
Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X, Liu NF. Programmed cell death in atherosclerosis and vascular calcification. Cell death & disease. 2022 May 18:13(5):467. doi: 10.1038/s41419-022-04923-5. Epub 2022 May 18 [PubMed PMID: 35585052]
Mirzayans R, Murray D. Do TUNEL and Other Apoptosis Assays Detect Cell Death in Preclinical Studies? International journal of molecular sciences. 2020 Nov 29:21(23):. doi: 10.3390/ijms21239090. Epub 2020 Nov 29 [PubMed PMID: 33260475]
Sorice M. Crosstalk of Autophagy and Apoptosis. Cells. 2022 Apr 28:11(9):. doi: 10.3390/cells11091479. Epub 2022 Apr 28 [PubMed PMID: 35563785]
Crowley LC, Marfell BJ, Waterhouse NJ. Detection of DNA Fragmentation in Apoptotic Cells by TUNEL. Cold Spring Harbor protocols. 2016 Oct 3:2016(10):. doi: 10.1101/pdb.prot087221. Epub 2016 Oct 3 [PubMed PMID: 27698233]
Edgington-Mitchell LE, Bogyo M. Detection of Active Caspases During Apoptosis Using Fluorescent Activity-Based Probes. Methods in molecular biology (Clifton, N.J.). 2016:1419():27-39. doi: 10.1007/978-1-4939-3581-9_3. Epub [PubMed PMID: 27108429]
Gomes MT, Palasiewicz K, Gadiyar V, Lahey K, Calianese D, Birge RB, Ucker DS. Phosphatidylserine externalization by apoptotic cells is dispensable for specific recognition leading to innate apoptotic immune responses. The Journal of biological chemistry. 2022 Jul:298(7):102034. doi: 10.1016/j.jbc.2022.102034. Epub 2022 May 16 [PubMed PMID: 35588784]
Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. The Journal of biological chemistry. 2001 Apr 13:276(15):12030-4 [PubMed PMID: 11134038]
Lossi L. The concept of intrinsic versus extrinsic apoptosis. The Biochemical journal. 2022 Feb 11:479(3):357-384. doi: 10.1042/BCJ20210854. Epub [PubMed PMID: 35147165]
Kashyap D, Garg VK, Goel N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Advances in protein chemistry and structural biology. 2021:125():73-120. doi: 10.1016/bs.apcsb.2021.01.003. Epub 2021 Apr 15 [PubMed PMID: 33931145]
Level 3 (low-level) evidenceWarren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell death & disease. 2019 Feb 21:10(3):177. doi: 10.1038/s41419-019-1407-6. Epub 2019 Feb 21 [PubMed PMID: 30792387]
Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clinical and experimental pharmacology & physiology. 2004 Mar:31(3):119-28 [PubMed PMID: 15008953]
Lee SB, Lee S, Park JY, Lee SY, Kim HS. Induction of p53-Dependent Apoptosis by Prostaglandin A(2). Biomolecules. 2020 Mar 24:10(3):. doi: 10.3390/biom10030492. Epub 2020 Mar 24 [PubMed PMID: 32213959]
Baena-Lopez LA, Wang L, Wendler F. Cellular stress management by caspases. Current opinion in cell biology. 2024 Feb:86():102314. doi: 10.1016/j.ceb.2023.102314. Epub 2024 Jan 11 [PubMed PMID: 38215516]
Level 3 (low-level) evidenceHu SJ, Jiang SS, Zhang J, Luo D, Yu B, Yang LY, Zhong HH, Yang MW, Liu LY, Hong FF, Yang SL. Effects of apoptosis on liver aging. World journal of clinical cases. 2019 Mar 26:7(6):691-704. doi: 10.12998/wjcc.v7.i6.691. Epub [PubMed PMID: 30968034]
Level 3 (low-level) evidenceNguyen TT, Wei S, Nguyen TH, Jo Y, Zhang Y, Park W, Gariani K, Oh CM, Kim HH, Ha KT, Park KS, Park R, Lee IK, Shong M, Houtkooper RH, Ryu D. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Experimental & molecular medicine. 2023 Aug:55(8):1595-1619. doi: 10.1038/s12276-023-01046-5. Epub 2023 Aug 23 [PubMed PMID: 37612409]
Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009 Sep 4:138(5):838-54. doi: 10.1016/j.cell.2009.08.021. Epub [PubMed PMID: 19737514]
Michie J, Kearney CJ, Hawkins ED, Silke J, Oliaro J. The Immuno-Modulatory Effects of Inhibitor of Apoptosis Protein Antagonists in Cancer Immunotherapy. Cells. 2020 Jan 14:9(1):. doi: 10.3390/cells9010207. Epub 2020 Jan 14 [PubMed PMID: 31947615]
Duarte-Olivenza C, Moran G, Hurle JM, Lorda-Diez CI, Montero JA. Lysosomes, caspase-mediated apoptosis, and cytoplasmic activation of P21, but not cell senescence, participate in a redundant fashion in embryonic morphogenetic cell death. Cell death & disease. 2023 Dec 9:14(12):813. doi: 10.1038/s41419-023-06326-6. Epub 2023 Dec 9 [PubMed PMID: 38071330]
Nössing C, Ryan KM. 50 years on and still very much alive: 'Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics'. British journal of cancer. 2023 Feb:128(3):426-431. doi: 10.1038/s41416-022-02020-0. Epub 2022 Nov 11 [PubMed PMID: 36369364]
Yin S, Ji C, Wu P, Jin C, Qian H. Human umbilical cord mesenchymal stem cells and exosomes: bioactive ways of tissue injury repair. American journal of translational research. 2019:11(3):1230-1240 [PubMed PMID: 30972158]
Level 2 (mid-level) evidenceMehrbod P, Ande SR, Alizadeh J, Rahimizadeh S, Shariati A, Malek H, Hashemi M, Glover KKM, Sher AA, Coombs KM, Ghavami S. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019 Dec:10(1):376-413. doi: 10.1080/21505594.2019.1605803. Epub [PubMed PMID: 30966844]
Kerr JF,Wyllie AH,Currie AR, Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British journal of cancer. 1972 Aug; [PubMed PMID: 4561027]
Level 3 (low-level) evidenceMcBride A, Houtmann S, Wilde L, Vigil C, Eischen CM, Kasner M, Palmisiano N. The Role of Inhibition of Apoptosis in Acute Leukemias and Myelodysplastic Syndrome. Frontiers in oncology. 2019:9():192. doi: 10.3389/fonc.2019.00192. Epub 2019 Mar 27 [PubMed PMID: 30972300]
Çıkla-Süzgün P, Küçükgüzel ŞG. Recent Advances in Apoptosis: THE Role of Hydrazones. Mini reviews in medicinal chemistry. 2019:19(17):1427-1442. doi: 10.2174/1389557519666190410125910. Epub [PubMed PMID: 30968776]
Level 3 (low-level) evidenceAngelousi A, Kassi E, Ansari-Nasiri N, Randeva H, Kaltsas G, Chrousos G. Clock genes and cancer development in particular in endocrine tissues. Endocrine-related cancer. 2019 Jun:26(6):R305-R317. doi: 10.1530/ERC-19-0094. Epub [PubMed PMID: 30959483]
Kabra UD, Jastroch M. Mitochondrial Dynamics and Insulin Secretion. International journal of molecular sciences. 2023 Sep 7:24(18):. doi: 10.3390/ijms241813782. Epub 2023 Sep 7 [PubMed PMID: 37762083]
Zhang S, Tang MB, Luo HY, Shi CH, Xu YM. Necroptosis in neurodegenerative diseases: a potential therapeutic target. Cell death & disease. 2017 Jun 29:8(6):e2905. doi: 10.1038/cddis.2017.286. Epub 2017 Jun 29 [PubMed PMID: 28661482]
Ge ZD, Lian Q, Mao X, Xia Z. Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy. International heart journal. 2019 May 30:60(3):512-520. doi: 10.1536/ihj.18-476. Epub 2019 Apr 10 [PubMed PMID: 30971629]
D'Addio F, Montefusco L, Lunati ME, Pastore I, Assi E, Petrazzuolo A, Marin V, Bruckmann C, Fiorina P. Targeting a novel apoptotic pathway in human disease. BioEssays : news and reviews in molecular, cellular and developmental biology. 2023 Jun:45(6):e2200231. doi: 10.1002/bies.202200231. Epub 2023 Mar 30 [PubMed PMID: 36998110]