Introduction
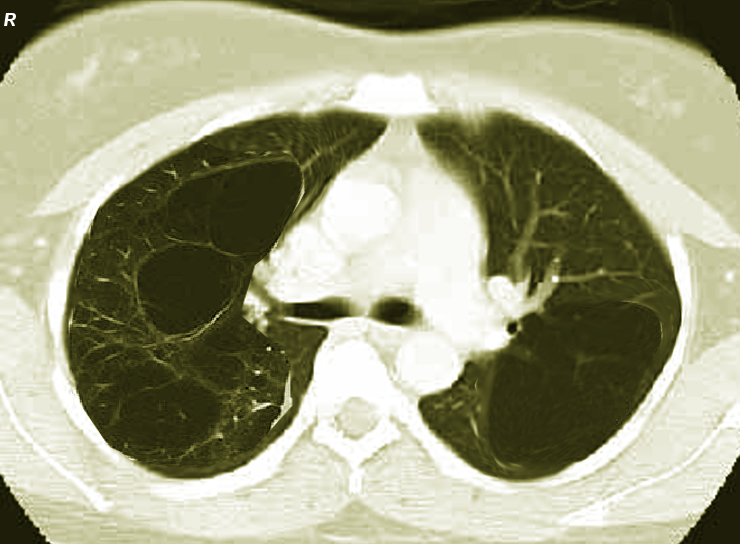
Emphysema is a form of chronic obstructive pulmonary disease (COPD) marked by airflow limitation due to the irreversible destruction and enlargement of alveolar spaces beyond the terminal bronchioles.[1] This loss of distal lung architecture often leads to bullae formation, an air-filled space larger than 1 cm, resulting from damaged lung parenchyma (see Image. Bilateral Bullous Emphysema, Computed Tomography). Approximately 80% of patients who present with bullae also have emphysema, and though terminology varies, the condition is often called bullous emphysema. Alternative terms like emphysematous bullae, giant emphysematous bullae, and bullous lung diseases are also common.[2] A giant bullae encompasses 30% or more of a hemithorax. The standard classifications of bullous emphysema are as follows:
- Type I: Isolated bullae without widespread emphysema
- Type II: Subpleural bullae
- Type III: Widespread bullae throughout the lung [2]
Emphysema is often sub-classified into groups based on the primary location of the emphysematous disease within the lung and acinus, a cluster of alveoli supplied by a single respiratory bronchiole. An acinus in the lungs is the smallest functional unit of the respiratory system. Proximal acinar or centrilobular emphysema refers to the destruction of the central portion of the acinus, which has general associations with smoking. Panacinar emphysema destroys all parts of the acinus and is usually related to α-1 antitrypsin deficiency.[3] Distal acinar or paraseptal emphysema involves the alveolar ducts and often occurs in combination with the previously listed forms of emphysema.[4]
Patients with COPD often present with progressive shortness of breath, productive cough, wheezing, and reduced exercise tolerance, though symptoms may develop gradually and remain unnoticed until later stages. Physical exam findings may include signs of lung hyperinflation, diminished breath sounds, wheezing, and signs of right heart strain. Pulmonary function tests are the cornerstone in establishing the diagnosis of emphysema, with chest radiography and laboratory testing such as a complete blood count, serum electrolytes, renal function, and thyroid function studies carried out to exclude other causes of dyspnea. Computed tomography may be necessary in patients with suspected complications, who are due for lung cancer screening, or if healthcare professionals are unable to distinguish bullae from a pneumothorax.
The management of bullous emphysema centers around smoking cessation and optimizing medical management. Patients who continue to have persistent symptoms or have giant bullae encompassing 30% or more of a hemithorax may require surgical intervention. Potential surgical interventions include lung volume reduction surgery, bullectomy, and placement of one-way valves using a bronchoscope, which allows trapped air to escape and reduces lung volumes.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The 2 most common causes of emphysema are smoking and α-1 antitrypsin deficiency, an inherited autosomal codominant genetic condition affecting the lungs, liver, and sometimes the skin.[5][6] Most commonly, smoking and COPD cause bullae formation, though some cases are idiopathic.[7]
Additionally, less common causes of emphysema and bullae unrelated to tobacco use include the following:
- Intravenous substance abuse leading to inflammatory or destructive damage to the alveoli
- Electronic cigarettes
- Smoking marijuana or crack cocaine
- Human immunodeficiency virus infection
- Pneumocystis carinii pneumonia
- Urticarial vasculitis syndrome with hypocomplementemia, a combination of urticaria, arthralgia, and angioedema, associated with panacinar emphysema
- Malnutrition-associated elastase-induced peripheral emphysema
- Sialic acid storage or Salla disease with impaired removal of sialic acid from lysosomes, causing cognitive impairment, ataxia, nystagmus, and basal and centriacinar emphysema
- Marfan syndrome
- Polyangiitis with granulomatosis
- Sjögren disease
- Sarcoidosis
- Ehlers-Danlos type IV
- COVID-19 infection [8][9][10][11][12][13][14]
Epidemiology
Emphysema is associated with high mortality and places a significant burden on healthcare systems due to frequent exacerbations, medical visits, and hospitalizations. According to the World Health Organization, COPD ranks as the fourth leading cause of death worldwide, with 3.5 million deaths in 2021, or about 5% of all global deaths. COPD affects more than 200 million people globally and primarily impacts older adults, as lung tissue damage is often associated with smoking and other age-related risk factors.[15]
Nearly 90% of COPD-related deaths in people younger than 70 occur in low- and middle-income countries.[16] While tobacco smoking causes over 70% of cases of COPD in high-income countries, this condition accounts for 30% to 40% in low- and middle-income countries, due to environmental exposures like household air pollution. Historically, emphysema has been more common in males. However, in developing countries, increased exposure to biomass fuel has led to a more equal distribution of risk between males and females.
Pathophysiology
Chronic inflammation of the distal air spaces, most often caused by harmful exposures like cigarette smoke, underlie the pathophysiology of bullous emphysema.[16] This inflammation leads to the destruction of alveolar walls and the permanent enlargement of air spaces. After an insult upon this barrier, such as by cigarette smoke, the resulting inflammatory response transports antigens to the bronchial-associated lymphatic tissue layer. The macrophages and neutrophils release enzymes such as elastase, which eventually destroy the lung's epithelial barrier.
As the alveolar walls are damaged, the air spaces become larger and less efficient. Over time, these changes impair gas exchange and reduce airflow due to a loss of elastic recoil in the lungs. Additional features include an increase in goblet cells, mucous gland hyperplasia, fibrosis, and airway collapse due to the loss of alveolar support. These changes contribute to chronic hypoxia and hypercarbia as the disease progresses.
History and Physical
Patients with COPD typically present with progressive shortness of breath, especially on exertion. They may also report a productive cough with thick sputum, often described as a “smoker’s cough,” which is commonly worse in the morning. Other common symptoms include wheezing, fatigue, breathlessness, reduced exercise tolerance, and, in some cases, changes in mental status. Some individuals may be asymptomatic in the early stages, and many do not present until late in the disease, given the gradual onset of symptoms. Patients with giant bullae may also present with chest pain and hemoptysis.
During a physical assessment, signs of hyperinflation may be observed, such as hyperresonance on chest percussion. The examination may also reveal tachypnea, diminished breath sounds, a barrel-shaped chest, wheezing, basilar crackles, or distant heart sounds. In addition, a pronounced P2 heart sound may also be evident, suggesting pulmonary hypertension. Cyanosis or edema in the extremities can occur due to reduced output from the right ventricle or cor pulmonale. Clubbing of the fingers and toes is uncommon unless additional conditions like pulmonary fibrosis, lung cancer, or bronchiectasis are present.[17]
Evaluation
Pulmonary function testing (PFT) is necessary to establish the diagnosis of COPD. PFTs confirm the presence of airflow obstruction, establish the severity, and monitor the progression of the disease.[18] The forced expiratory volume in 1 second/forced vital capacity ratios will typically be less than 0.7 and incompletely reversible after administering an inhaled bronchodilator, as assessed by spirometry following bronchodilator use. Forced vital capacity decreases because of the loss of elastic lung recoil. Additional expected findings are an increased total lung capacity (TLC), residual volume (RV), and functional residual capacity.
The air trapping identified on PFTs, manifesting as an RV/TLC ratio elevation, corresponds to emphysematous changes found on computed tomography scans of the chest, particularly during expiration.[19] Likewise, TLC can predict hyperinflation and emphysema when increased by more than 120% of predicted in individuals who have a history of smoking.[20] A decrease in the diffusing capacity of the lungs for carbon monoxide will often be present because of the destruction of the lung interstitium. However, normal PFTs are common in individuals with lung disease or symptoms, risk factors, a category classified by the Global Initiative for Obstructive Lung Disease as class 0.[21] Hence, clinical and radiological assessments are essential in these cases to overcome these patients' significant under-estimation and late identification.
Healthcare professionals should test for α-1 antitrypsin deficiency in patients with emphysema and persistent airflow obstruction.[3] Additional clinical clues that should prompt testing are:
- Emphysema in a patient aged 45 years or younger or who has never smoked
- Predominantly basilar changes on chest radiograph in a patient with emphysema
- Patients with emphysema and a family history of emphysema or liver disease
- Adult-onset asthma that fails to improve with bronchodilators
- Current findings or a history of panniculitis
- Patients with emphysema and unexplained chronic liver disease
Patients with suspected COPD undergo plain chest radiographs, which often reveal a flattened diaphragm because of hyperinflation. Bullae appear as radiolucent areas surrounded by thin, curved, white, or light-colored lines representing interlobar fissures. As the disease progresses, prominent hilar vascular markings and cardiomegaly may be evident.[22] Computed tomography (CT) scan of the chest is more sensitive for detecting COPD than chest radiography. A CT scan of the chest is useful when clinicians suspect a complication of COPD or an alternative diagnosis like pneumonia, pneumothorax, giant bullae, thromboembolic disease, or lung cancer, or if a lung cancer screen is appropriate.[23][24][25] Giant bullae are typically evident on chest radiographs. However, they may be difficult to differentiate from a pneumothorax. A CT scan of the chest is helpful in these circumstances.
To evaluate for other potential etiologies of dyspnea, patients should undergo a complete blood count, serum electrolytes and kidney function, thyroid stimulating hormone level, and plasma brain natriuretic peptide (BNP) or N-terminal pro-BNP to assess for anemia, kidney disease, thyroid disorders, or heart failure. Electrocardiography may show right axis deviation, right ventricular hypertrophy, or right atrial hypertrophy. Expected findings due to COPD may be increased hemoglobin and hematocrit due to a reactive erythrocytosis secondary to chronic hypoxia. In addition, eosinophilia can indicate a type II inflammation phenotype and overlap with asthma.[26] The eosinophil count can serve as a potential biomarker and assist in stratifying patients into treatable groups.[27]
Patients may have an elevated serum bicarbonate level due to metabolic compensation for respiratory acidosis. Vitamin D levels have been studied extensively in individuals with COPD and reveal that severe vitamin D deficiency can predict frequent exacerbation and hospitalizations.[28][29] Hence, clinicians should assess vitamin D levels in individuals with emphysema, especially those treated with corticosteroids or with an increased risk of bone disease.[30][31] Echocardiography helps to assess right ventricular systolic pressures and estimate the presence of pulmonary hypertension.[32] In addition, clinicians can estimate pulmonary artery pressure by measuring the pulmonary artery (PA) to the aorta (A) ratio on a chest CT scan. A PA/A ratio greater than 1 indicates pulmonary hypertension.[33]
In addition, clinical assessment classification, based on indexes such as the Body Mass Index Airflow Obstruction, Dyspnea, and Exercise Capacity (BODE) index, helps predict mortality and risk of hospitalization. Clinicians calculate the BODE index based on the body mass index, forced expiratory volume in 1 second, the Medical Research Council dyspnea score, and six-minute walk distance.[34] The COPD Assessment Test score is a good measure of the impact of COPD on a patient's life, particularly in response to rehabilitation or after exacerbations. A decrease in score of 2 points is a clinically significant improvement concerning a patient's health status and symptoms.[35]
Treatment / Management
Chronic Medical Therapy for Non α-1 Antitrypsin Deficiency Bullous Emphysema
Chronic therapy includes several interventions similar to those used to manage COPD without bullous emphysema.[36][37] Pulmonary rehabilitation improves patients' quality of life and pulmonary function while decreasing healthcare utilization through exercise training, education, and behavioral change. Clinicians base chronic inhaled therapy on the classification of disease and symptoms outlined by the most recent Global Initiative for Chronic Obstructive Lung Disease guidelines.[36](B3)
Initially, all patients should receive a short-acting β-agonist (SABA) such as albuterol for acute shortness of breath and bronchodilation. Following the SABA rescue bronchodilator, patients receive a long-acting muscarinic antagonist (LAMA) and continue the SABA as needed or use a long-acting β-agonist (LABA)-LAMA combination with a SABA as needed based on their symptom severity. Patients with poor symptom control, with at least 1 hospitalization, 2 or more moderate exacerbations over the previous year, or high peripheral eosinophil levels may require inhaled corticosteroids in addition to the LAMA-LABA combination. See StatPearl's companion topics, "Chronic Obstructive Pulmonary Disease and Emphysema'" for further discussion regarding medication management of COPD. Long-term oxygen is a possible add-on therapy if the pO2 is below 55 mm Hg or the oxygen saturation is less than 88% on a 6-minute walk test. For emphysema due to α-1 antitrypsin deficiency, clinicians use augmentation therapy.[38]
Preventive Care
Smoking cessation is of critical importance to combat disease progression and decrease mortality. Necessary vaccinations for patients with COPD include the following:
- Annual influenza vaccine
- Pneumococcal vaccine based on local guidelines. In the United States and Canada, the current guidelines are the pneumococcal conjugate vaccine (PCV) 21, except for residents of the Navajo Nation or individuals who reside in the Western United States and Canada who have substance use disorder or who experience homelessness. These patients should receive PCV20 or PCV15, followed by the pneumococcal polysaccharide vaccine (PPSV23) due to the lack of serotype 4 in PCV21. Further guidelines regarding recommendations for revaccinations and patients who have previously received PPSV23 are available through the United States Centers for Disease Control at Expanded Recommendations for Use of Pneumococcal Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, 2024.
- Pertussis
- COVID-19
- Respiratory syncytial virus in those aged 60 or older with COPD
- Shingles or herpes zoster
Many patients with COPD will experience pulmonary cachexia, a body mass index of 20 kg/m2 or less, or a weight below the 90% percentile of the patient's ideal body weight, secondary to the increased metabolic demands of the increased work of breathing. Patients exhibiting these findings will require nutritional support.[39]
Acute Exacerbations
Patients experiencing an acute exacerbation should receive oxygen titrated to an oxygen saturation of 88% to 92%, albuterol-ipratropium, and steroids. Systemic glucocorticoid therapy with a short 5-day course of prednisone provides benefits. Patients with any 2 of the following: increased dyspnea, increased sputum production, viscosity, or purulence, plus an additional risk factor for a poor outcome, also warrant antibiotic therapy. Patients in the hospital are frequently monitored with arterial blood gas to assess for carbon dioxide retention. Understanding potential triggers, like respiratory infections, is also important in managing acute and preventing future COPD exacerbations.
Additional Interventions
Following smoking cessation and optimization of medical therapy, patients who remain symptomatic may require surgical intervention. The general indications include moderate or severe dyspnea, giant bullae, and complications such as pneumothorax, infection, and hemoptysis. Where medical management is inadequate, surgical interventions such as lung volume reduction surgery (LVRS), bullectomy, and lung transplantation are necessary.[40] Surgeons remove one or more giant bullae by thoracotomy or video-assisted thoracoscopic surgery. LVRS involves a wedge excision of emphysematous tissue.
Experts believe reducing the size of hyperinflated lungs improves expiratory airflow in the non-diseased lungs. Study results reveal that elastic recoil of the lungs may improve after LVRS, which improves the expiratory airflow in the lungs. Affected patients must meet extensive criteria before undergoing LVRS, including notable airflow obstruction on spirometry, evidence of air trapping on lung volume measurements, and CT findings of heterogeneously distributed emphysema.[41][42]
A more recent minimally invasive treatment, using a one-way valve, placed via bronchoscopy, results in lung volume reduction by allowing trapped air and secretions within a lobe to escape but no new air or secretions to re-enter. Indications for the procedure are evidence of emphysema on CT scan with intact lobar fissures, progressive dyspnea as indicated by a Modified Medical Research Council of 2 or more, smoking cessation of 4 months or more, severe airflow obstruction measured on PFTS as an forced expiratory volume in 1 second (FEV1) less than 45% with air trapping as evidenced by an RV of 150% to 170%, and a 6-minute walk test of at least 100 meters.
Contraindications for bronchoscopic valve placement include obesity or a body mass index of more than 35 kg/m2, congestive heart failure with an ejection fraction of less than 40%, prior thoracic surgery, limited mobility or a 6-minute walk test of fewer than 100 meters, severe hypoxic or hypercapnic respiratory failure with a partial pressure of oxygen less than 45 mm Hg or partial pressure of carbon dioxide in arterial blood greater than 60 mm Hg, active infection, and a severely reduced FEV1 of less than 15%. Patients undergoing these treatments have shown a 100 to 200 mL improvement in their FEV1 and subjective symptoms due to decreased air trapping. However, the risk of pneumothorax is 25% to 30 %.[43]
Differential Diagnosis
The following list includes the potential differential diagnoses for bullous emphysema:
- Asthma
- Bronchiectasis
- Idiopathic bullous lung disease
- Bullous lung disease due to human immunodeficiency virus infection
- Bullous lung disease due to intravenous drug use
- α-1 antitrypsin deficiency
- Birt-Hogg-Dubé syndrome
- Malignancy
- Lymphangioleiomyomatosis
- Chronic bronchitis
- Airway obstruction
- Heart failure
- Tuberculosis
- Interstitial lung disease
- Thromboembolic disease
- Pulmonary Langerhans cell histiocytosis [38][39][40]
Complications
Complications related to bullous emphysema arise from both the disease and treatment. The following list includes potential complications related to bullous emphysema:
- Pneumothorax
- Pneumonia and other respiratory infections
- Respiratory failure
- Cor pulmonale
- Pulmonary hypertension
- Myocardial infarction
- Osteoporosis due to glucocorticoids and inactivity
- Muscle weakness
- Cachexia
- Anxiety and depression
- Complications due to LVRS include death, arrhythmia, air leak, deep vein thrombosis, pulmonary embolism, and wound infection [41][42][43][44][45][46][47][48][49]
Deterrence and Patient Education
Bullous emphysema, a form of COPD characterized by the irreversible destruction of alveolar structures resulting in the formation of bullae, places a significant burden on healthcare systems and dramatically impacts patients' lives. Patient education plays a vital role in preventing and managing bullous emphysema. Smoking cessation remains the most important modifiable intervention and should be strongly encouraged. Educating patients about the risks of environmental and occupational exposures, including air pollutants and biomass smoke, is also essential in reducing disease progression.
Clinicians should educate patients about the nature of their condition and the associated risks, including pneumothorax, heart failure, myocardial infarction, pneumonia, and increased mortality. Awareness of these potential complications helps patients appreciate the need for recommended diagnostic evaluations and highlights the importance of consistent medication use and preventive care. Reinforcing the value of maintenance therapies, routine vaccinations, pulmonary rehabilitation, and regular follow-up can empower patients to participate in their treatment actively, ultimately improving outcomes and reducing the likelihood of serious complications. Clinicians should counsel patients on recognizing early signs of worsening disease—such as increased dyspnea, purulent sputum, chest discomfort, or hemoptysis—and when to seek medical care. Following smoking cessation and medication optimization, patients with persistent symptoms or giant bullae may require surgical intervention.
Enhancing Healthcare Team Outcomes
Bullous emphysema is a form of COPD characterized by airflow limitation due to irreversible destruction and enlargement of alveolar spaces beyond the terminal bronchioles. This structural damage can lead to the formation of bullae within the lung parenchyma. Clinically, patients may present with progressive dyspnea, wheezing, productive cough, and exercise intolerance, though symptoms may be insidious. Physical findings include signs of hyperinflation, diminished breath sounds, and possible indicators of right heart strain. Diagnosis is typically confirmed with pulmonary function testing, supported by imaging and lab studies to exclude other causes of dyspnea. CT imaging is particularly valuable in distinguishing bullae from pneumothorax or evaluating for complications. Management focuses on smoking cessation and optimizing medical therapy. In patients with persistent symptoms or giant bullae, surgical options such as lung volume reduction surgery, bullectomy, or bronchoscopic one-way valve placement are potential options.
Effective management of bullous emphysema requires a coordinated, multidisciplinary approach in which each healthcare professional contributes specific skills and collaborates to deliver patient-centered, evidence-based care. Advanced clinicians must thoroughly understand the pathophysiology, diagnostic criteria, and treatment options for bullous emphysema, including the ability to interpret pulmonary function tests and imaging to determine disease severity and implement appropriate therapy. Nurses play a key role in patient education, symptom monitoring, and early recognition of exacerbations or complications such as pneumothorax while supporting adherence to medication and oxygen therapy.
Pharmacists contribute by ensuring appropriate medication management, identifying potential drug interactions, and counseling patients on the correct use of inhalers and other therapies. Respiratory therapists are instrumental in delivering pulmonary rehabilitation and teaching breathing techniques that improve lung efficiency and quality of life. Each healthcare team member must communicate findings clearly and consistently with the broader care team to inform timely decision-making. Interprofessional communication is essential for coordinating care, especially when considering surgical interventions.
A successful strategy includes regular team meetings, shared electronic health records, and clearly defined care pathways. Care coordination ensures continuity across settings—from outpatient clinics to surgical consults and post-discharge follow-up—helping to prevent hospital readmissions and improve long-term outcomes. When healthcare teams align their expertise and maintain open communication, they enhance patient safety, improve treatment outcomes, and empower patients to participate in their care actively.
Media
(Click Image to Enlarge)
References
Centers for Disease Control and Prevention (CDC). Chronic obstructive pulmonary disease among adults--United States, 2011. MMWR. Morbidity and mortality weekly report. 2012 Nov 23:61(46):938-43 [PubMed PMID: 23169314]
Teramoto S, Fukuchi Y. Bullous emphysema. Current opinion in pulmonary medicine. 1996 Mar:2(2):90-6 [PubMed PMID: 9363122]
Level 3 (low-level) evidenceJanssen R, Piscaer I, Franssen FME, Wouters EFM. Emphysema: looking beyond alpha-1 antitrypsin deficiency. Expert review of respiratory medicine. 2019 Apr:13(4):381-397. doi: 10.1080/17476348.2019.1580575. Epub 2019 Feb 22 [PubMed PMID: 30761929]
Wang K, Pan T, Yang H, Ruan W, Zhong J, Wu G, Zhou X. Assessment of pulmonary microstructural changes by hyperpolarized (129)Xe diffusion-weighted imaging in an elastase-instilled rat model of emphysema. Journal of thoracic disease. 2017 Aug:9(8):2572-2578. doi: 10.21037/jtd.2017.08.39. Epub [PubMed PMID: 28932564]
Gaensler EA, Jederlinic PJ, FitzGerald MX. Patient work-up for bullectomy. Journal of thoracic imaging. 1986 Mar:1(2):75-93 [PubMed PMID: 3599138]
Perkins JT, Choate R, Mannino DM, Browning SR, Sandhaus RA. Benefits Among Patients with Alpha-1 Antitrypsin Deficiency Enrolled in a Disease Management and Prevention Program. Chronic obstructive pulmonary diseases (Miami, Fla.). 2016 Dec 24:4(1):56-64. doi: 10.15326/jcopdf.4.1.2016.0161. Epub 2016 Dec 24 [PubMed PMID: 28848911]
Thurlbeck WM. Pathophysiology of chronic obstructive pulmonary disease. Clinics in chest medicine. 1990 Sep:11(3):389-403 [PubMed PMID: 2205438]
Kalra SS, Pais F, Harman E, Urbine D. Rapid development of bullous lung disease: a complication of electronic cigarette use. Thorax. 2020 Apr:75(4):359. doi: 10.1136/thoraxjnl-2019-214333. Epub 2020 Feb 10 [PubMed PMID: 32041740]
Tashkin DP. Marijuana and Lung Disease. Chest. 2018 Sep:154(3):653-663. doi: 10.1016/j.chest.2018.05.005. Epub 2018 May 17 [PubMed PMID: 29778658]
de Almeida RR, de Souza LS, Mançano AD, Souza AS Jr, Irion KL, Nobre LF, Zanetti G, Hochhegger B, Pereira e Silva JL, Marchiori E. High-resolution computed tomographic findings of cocaine-induced pulmonary disease: a state of the art review. Lung. 2014 Apr:192(2):225-33. doi: 10.1007/s00408-013-9553-6. Epub 2014 Jan 16 [PubMed PMID: 24429586]
Goldstein DS, Karpel JP, Appel D, Williams MH Jr. Bullous pulmonary damage in users of intravenous drugs. Chest. 1986 Feb:89(2):266-9 [PubMed PMID: 3943387]
Lee P, Gildea TR, Stoller JK. Emphysema in nonsmokers: alpha 1-antitrypsin deficiency and other causes. Cleveland Clinic journal of medicine. 2002 Dec:69(12):928-9, 933, 936 passim [PubMed PMID: 12546267]
Rosen MJ, Lou Y, Kvale PA, Rao AV, Jordan MC, Miller A, Glassroth J, Reichman LB, Wallace JM, Hopewell PC. Pulmonary function tests in HIV-infected patients without AIDS. Pulmonary Complications of HIV Infection Study Group. American journal of respiratory and critical care medicine. 1995 Aug:152(2):738-45 [PubMed PMID: 7633736]
Wood JR, Bellamy D, Child AH, Citron KM. Pulmonary disease in patients with Marfan syndrome. Thorax. 1984 Oct:39(10):780-4 [PubMed PMID: 6495247]
Sobonya RE, Burrows B. The epidemiology of emphysema. Clinics in chest medicine. 1983 Sep:4(3):351-8 [PubMed PMID: 6357599]
Kemp SV, Polkey MI, Shah PL. The epidemiology, etiology, clinical features, and natural history of emphysema. Thoracic surgery clinics. 2009 May:19(2):149-58. doi: 10.1016/j.thorsurg.2009.03.003. Epub [PubMed PMID: 19662957]
Kishaba T, Shimaoka Y, Fukuyama H, Yoshida K, Tanaka M, Yamashiro S, Tamaki H. A cohort study of mortality predictors and characteristics of patients with combined pulmonary fibrosis and emphysema. BMJ open. 2012:2(3):. doi: 10.1136/bmjopen-2012-000988. Epub 2012 May 15 [PubMed PMID: 22587885]
Ponce MC, Sankari A, Sharma S. Pulmonary Function Tests. StatPearls. 2025 Jan:(): [PubMed PMID: 29493964]
Eda S, Kubo K, Fujimoto K, Matsuzawa Y, Sekiguchi M, Sakai F. The relations between expiratory chest CT using helical CT and pulmonary function tests in emphysema. American journal of respiratory and critical care medicine. 1997 Apr:155(4):1290-4 [PubMed PMID: 9105069]
Kinsella M, Müller NL, Staples C, Vedal S, Chan-Yeung M. Hyperinflation in asthma and emphysema. Assessment by pulmonary function testing and computed tomography. Chest. 1988 Aug:94(2):286-9 [PubMed PMID: 3396405]
Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, Kauczor HU, Bailey WC, DeMeo DL, Casaburi RH, Friedman P, Van Beek EJ, Hokanson JE, Bowler RP, Beaty TH, Washko GR, Han MK, Kim V, Kim SS, Yagihashi K, Washington L, McEvoy CE, Tanner C, Mannino DM, Make BJ, Silverman EK, Crapo JD, Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA internal medicine. 2015 Sep:175(9):1539-49. doi: 10.1001/jamainternmed.2015.2735. Epub [PubMed PMID: 26098755]
Matthay RA, Niederman MS, Wiedemann HP. Cardiovascular-pulmonary interaction in chronic obstructive pulmonary disease with special reference to the pathogenesis and management of cor pulmonale. The Medical clinics of North America. 1990 May:74(3):571-618 [PubMed PMID: 2186234]
Wong AW, Liang J, Cottin V, Ryerson CJ. Diagnostic Features in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review. Annals of the American Thoracic Society. 2020 Oct:17(10):1333-1336. doi: 10.1513/AnnalsATS.202002-122RL. Epub [PubMed PMID: 32610025]
Level 1 (high-level) evidenceHenschke CI, Yip R, Boffetta P, Markowitz S, Miller A, Hanaoka T, Wu N, Zulueta JJ, Yankelevitz DF, I-ELCAP Investigators. CT screening for lung cancer: Importance of emphysema for never smokers and smokers. Lung cancer (Amsterdam, Netherlands). 2015 Apr:88(1):42-7. doi: 10.1016/j.lungcan.2015.01.014. Epub 2015 Feb 4 [PubMed PMID: 25698134]
Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, Hirai T, Niimi A, Nishimura K, Chin K, Mishima M. CT scan findings of emphysema predict mortality in COPD. Chest. 2010 Sep:138(3):635-40. doi: 10.1378/chest.09-2836. Epub 2010 Apr 9 [PubMed PMID: 20382712]
Maniscalco M, Candia C, Ambrosino P, Iovine A, Fuschillo S. Chronic obstructive pulmonary disease's eosinophilic phenotype: Clinical characteristics, biomarkers and biotherapy. European journal of internal medicine. 2025 Jan:131():27-35. doi: 10.1016/j.ejim.2024.10.015. Epub 2024 Oct 22 [PubMed PMID: 39443246]
Nakamura S, Wakahara K, Majima S, Yokoi E, Fukutani E, Otsuki R, Iwano S, Chen-Yoshikawa TF, Kinoshita F, Abe T, Sashio T, Kimura T, Izuhara K, Hashimoto N, Ishii M, Hasegawa Y. Blood eosinophil count correlates with alveolar damage in emphysema-predominant COPD. BMC pulmonary medicine. 2024 Oct 13:24(1):510. doi: 10.1186/s12890-024-03320-2. Epub 2024 Oct 13 [PubMed PMID: 39396940]
Li B, Liu M, Wang Y, Zhang H, Xuan L, Huang K, An Z. Association of Severe Vitamin D Deficiency with Hospitalization in the Previous Year in Hospitalized Exacerbated COPD Patients. International journal of chronic obstructive pulmonary disease. 2024:19():1471-1478. doi: 10.2147/COPD.S461029. Epub 2024 Jun 25 [PubMed PMID: 38948911]
Malinovschi A, Masoero M, Bellocchia M, Ciuffreda A, Solidoro P, Mattei A, Mercante L, Heffler E, Rolla G, Bucca C. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respiratory research. 2014 Dec 13:15(1):131. doi: 10.1186/s12931-014-0131-0. Epub 2014 Dec 13 [PubMed PMID: 25496239]
Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. American journal of respiratory and critical care medicine. 2009 Apr 15:179(8):630-6. doi: 10.1164/rccm.200810-1576PP. Epub 2009 Jan 22 [PubMed PMID: 19164701]
Jaramillo JD, Wilson C, Stinson DS, Lynch DA, Bowler RP, Lutz S, Bon JM, Arnold B, McDonald ML, Washko GR, Wan ES, DeMeo DL, Foreman MG, Soler X, Lindsay SE, Lane NE, Genant HK, Silverman EK, Hokanson JE, Make BJ, Crapo JD, Regan EA, COPDGene Investigators. Reduced Bone Density and Vertebral Fractures in Smokers. Men and COPD Patients at Increased Risk. Annals of the American Thoracic Society. 2015 May:12(5):648-56. doi: 10.1513/AnnalsATS.201412-591OC. Epub [PubMed PMID: 25719895]
Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, Fessler HE, NETT Research Group. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. The European respiratory journal. 2007 Nov:30(5):914-21 [PubMed PMID: 17652313]
Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014 Apr:145(4):824-832. doi: 10.1378/chest.13-1422. Epub [PubMed PMID: 24114440]
Level 2 (mid-level) evidenceCelli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. The New England journal of medicine. 2004 Mar 4:350(10):1005-12 [PubMed PMID: 14999112]
Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, Haselden BM, Polkey MI, Man WD. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. The Lancet. Respiratory medicine. 2014 Mar:2(3):195-203. doi: 10.1016/S2213-2600(14)70001-3. Epub 2014 Feb 4 [PubMed PMID: 24621681]
Agusti A, Vogelmeier CF. GOLD 2024: a brief overview of key changes. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2023 Dec 22:49(6):e20230369. doi: 10.36416/1806-3756/e20230369. Epub 2023 Dec 22 [PubMed PMID: 38126685]
Level 3 (low-level) evidenceAgarwal AK, Raja A, Brown BD. Chronic Obstructive Pulmonary Disease. StatPearls. 2025 Jan:(): [PubMed PMID: 32644707]
Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse Cystic Lung Disease. Part I. American journal of respiratory and critical care medicine. 2015 Jun 15:191(12):1354-66. doi: 10.1164/rccm.201411-2094CI. Epub [PubMed PMID: 25906089]
Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse Cystic Lung Disease. Part II. American journal of respiratory and critical care medicine. 2015 Jul 1:192(1):17-29. doi: 10.1164/rccm.201411-2096CI. Epub [PubMed PMID: 25906201]
Asai N, Ohkuni Y, Matsunuma R, Nakashima K, Iwasaki T, Kaneko N. Infectious giant bulla associated with lung cancer. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2011 May-Jun:37(3):404-8 [PubMed PMID: 21755198]
Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005 Jun:127(6):1952-9 [PubMed PMID: 15947307]
Level 1 (high-level) evidenceOhara T, Hirai T, Muro S, Haruna A, Terada K, Kinose D, Marumo S, Ogawa E, Hoshino Y, Niimi A, Chin K, Mishima M. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008 Dec:134(6):1244-1249. doi: 10.1378/chest.07-3054. Epub 2008 Jul 18 [PubMed PMID: 18641115]
Park HY, Jhun BW, Jeong HJ, Chon HR, Koh WJ, Suh GY, Kim H, Chung MJ, Jun HJ, Choi YH, Lim SY. The complex association of metabolic syndrome and its components with computed tomography-determined emphysema index. Metabolic syndrome and related disorders. 2015 Apr:13(3):132-9. doi: 10.1089/met.2014.0117. Epub 2015 Jan 8 [PubMed PMID: 25569241]
Swallow EB, Barreiro E, Gosker H, Sathyapala SA, Sanchez F, Hopkinson NS, Moxham J, Schols A, Gea J, Polkey MI, ENIGMA in COPD project. Quadriceps muscle strength in scoliosis. The European respiratory journal. 2009 Dec:34(6):1429-35. doi: 10.1183/09031936.00074008. Epub 2009 May 14 [PubMed PMID: 19443534]
Kozora E, Emery C, Kaplan RM, Wamboldt FS, Zhang L, Make BJ. Cognitive and psychological issues in emphysema. Proceedings of the American Thoracic Society. 2008 May 1:5(4):556-60. doi: 10.1513/pats.200708-123ET. Epub [PubMed PMID: 18453371]
Renvall MJ, Friedman P, Ramsdell JW. Predictors of body mass index in patients with moderate to severe emphysema. COPD. 2009 Dec:6(6):432-6. doi: 10.3109/15412550903433034. Epub [PubMed PMID: 19938965]
Maddocks M, Lovell N, Booth S, Man WD, Higginson IJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet (London, England). 2017 Sep 2:390(10098):988-1002. doi: 10.1016/S0140-6736(17)32127-X. Epub [PubMed PMID: 28872031]
Naunheim KS, Wood DE, Krasna MJ, DeCamp MM Jr, Ginsburg ME, McKenna RJ Jr, Criner GJ, Hoffman EA, Sternberg AL, Deschamps C, National Emphysema Treatment Trial Research Group. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. The Journal of thoracic and cardiovascular surgery. 2006 Jan:131(1):43-53 [PubMed PMID: 16399293]
Level 1 (high-level) evidenceDeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, McKenna RJ Jr, NETT Research Group. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. The Annals of thoracic surgery. 2006 Jul:82(1):197-206; discussion 206-7 [PubMed PMID: 16798215]
Level 3 (low-level) evidence