Introduction
The electroencephalogram (EEG) is a noninvasive technique for measuring neurophysiological activity. This evaluation modality serves as a valuable tool for analyzing the brain's complex functions by detecting electrical activity. EEG signals reflect the brain’s electrical function and are recorded by placing electrodes on the scalp to measure cortical neuron activity.[1]
Hans Berger first used EEG in humans in 1924, publishing his findings in 1929. EEG records voltage fluctuations over time from multiple scalp electrodes arranged in a specific pattern to sample different cortical regions. These signals represent fluctuating dendritic potentials from superficial cortical layers, requiring voltage amplification for proper recording. However, extracranial electrodes do not effectively capture deep brain activity.
EEG waveforms result from the summation of excitatory (EPSPs) and inhibitory postsynaptic potentials (IPSPs) in cortical neurons. Some benign EEG variants appear normal but may be mistaken for epileptiform activity.[2] Abnormal EEG waveforms include both epileptiform and nonepileptiform abnormalities. Recognizing the differences requires understanding normal EEG patterns across different physiological states in children and adults. Electroencephalographers must also distinguish artifacts from true abnormalities and identify benign variants. This article reviews abnormal EEG waveforms and their clinical significance.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
EEG has several clinical applications, particularly in the evaluation of neurological disorders:
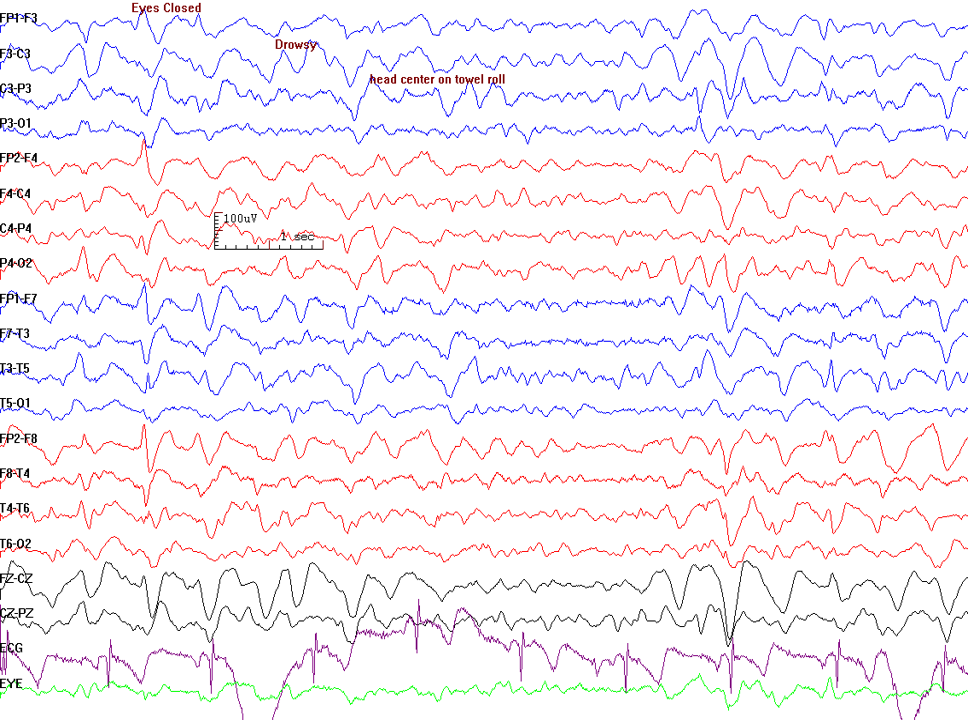
- Distinguishing epileptic seizures from psychogenic nonepileptic seizures, syncope (fainting), subcortical movement disorders, and migraine variants (see Image. Electroencephalogram Pattern for a Nonepileptic Seizure)
- Differentiating encephalopathy from psychiatric syndromes such as catatonia
- Assessing the need for discontinuing antiepileptic medications
- Characterizing seizures to guide the selection of appropriate antiepileptic therapy
- Localizing seizure onset zones to evaluate candidacy for epilepsy surgery
EEG is vital for epilepsy detection. Recent studies have introduced a novel class of convolutional neural networks (CNNs) designed to identify abnormal waveforms and sensor patterns in epilepsy-related EEGs. A deep 1-dimensional convolutional neural network (1D CNN) model has been proposed for the automated classification of normal, preictal, and seizure-related EEG patterns.
EEG has been excluded as an ancillary test for determining brain death by the American Academy of Neurology. The World Brain Death Project advises against routine EEG use in adults due to its inability to assess the function of deep brain structures and the brainstem. Brain death diagnosis primarily relies on clinical assessment, including the absence of brainstem reflexes and apnea testing. If performed, EEG should be interpreted according to established criteria (see Issues of Concern: Other Diffuse or Focal Abnormal Electroencephalogram Patterns).[3]
Issues of Concern
Some normal EEG waveforms may appear abnormal depending on various factors. For example, α waves, which typically appear over the posterior head regions in an awake individual as the posterior background rhythm, can take on pathological significance in certain comatose states. Diffuse α activity, known as α coma, strongly suggests severe brain dysfunction.
Current studies have identified abnormal EEG patterns in people with schizophrenia during rest, including increased phase discontinuity in the α- and θ-bands, heightened EEG power across multiple bands, and a reduced peak α frequency.[4] δ waves are normal in drowsiness and early childhood. However, focal δ activity can be abnormal (see below). β activity typically appears in the frontal regions and spreads posteriorly during early sleep. In some cases, focal β activity is associated with structural lesions and certain epilepsies, such as generalized fast activity (GFA). Medications like sedatives (eg, phenobarbital and benzodiazepines) commonly produce diffuse β activity.
Recent reviews have highlighted EEG abnormalities suggesting a genetic predisposition to epilepsy, including generalized spike-and-wave paroxysms, focal spikes and sharp waves, photosensitivity, 4- to 7-Hz θ rhythm, 2- to 4-Hz occipital intermittent rhythmic δ activity, φ rhythm, and generalized monomorphic α background activity. These EEG abnormalities may influence syndrome-specific phenotypes, predict disease severity and treatment resistance, and impact overall prognosis.[5]
Triphasic Waves
Triphasic waves were first described by Foley in 1950, and Bickford and Butt named them in 1955. Initially thought to be pathognomonic for hepatic encephalopathy, triphasic waves are now recognized as nonspecific findings that can occur in any metabolic encephalopathy. These high-amplitude sharp waves have 3 phases, each progressively shorter in duration (see Image. Triphasic Waves). The sharply contoured waveform always begins with a negative deflection, giving rise to the term "triphasic waves." Triphasic waves appear diffusely with a bifrontal predominance and are synchronous. These signals do not occur in the awake state but are observed in individuals with altered levels of consciousness. Triphasic waves are hypothesized to result from structural or metabolic abnormalities affecting thalamocortical circuits, likely due to disruptions in thalamocortical relay function.[6][7][8]
Triphasic waves have been described as "blunted spike waves." The main phase is surface-positive with high amplitude (>70 μV), preceded by a low-amplitude negative deflection and followed by a slow-rising, broad negative deflection. Recent studies classify triphasic waves as a subset of generalized periodic discharges, suggesting that these patterns result from an imbalance between cortical excitation and synaptic transmission within functionally connected networks, including the thalamocortical pathways involved in arousal.[9][10]
Other studies have identified white matter disease (WMD) as a contributor to triphasic waves, even in the absence of commonly cited triggers, such as hepatic disease, severe uremia beyond the baseline, medications like cefepime, ifosfamide, lithium, and baclofen, or global hypoxic-ischemic injury. Less recognized risk factors, including WMD, mild metabolic disturbances, or infectious processes, may also contribute to triphasic waves. As the older adult population grows, WMD prevalence is expected to rise, necessitating a broader differential diagnosis for triphasic waves.
Clinicians should consider not only metabolic abnormalities such as glucose dysregulation, calcium imbalances, and hypernatremia but also structural conditions like acute stroke and chronic central nervous system abscesses. Additionally, triphasic waves frequently cooccur with infections affecting the urinary tract, respiratory system, and central nervous system.[11]
Interictal Epileptiform Discharges
Interictal epileptiform discharge (IED) is an abnormal synchronous electrical discharge generated by a group of neurons within the epileptic focus.[12] These discharges represent the epileptic focus in individuals with seizures. Routine 30-minute EEG recordings have low sensitivity for detecting IEDs, but the diagnostic yield increases with repeated or prolonged EEG monitoring. In children with a new-onset seizure, the presence of IEDs in routine EEG ranges from 18% to 56%, while in adults, the detection rate varies from 12% to 50%.[13] Although uncommon, IEDs can also occur in individuals without a history of seizures.[14] IEDs are classified into spikes, polyspikes, and sharp waves.
Spike-and-wave complexes consist of a brief spike lasting 20 to 70 ms, followed by a slower wave component. The wave component is generated by currents mediated by γ-aminobutyric acid type B (GABA-B) receptors.[15] Polyspikes are characterized by 2 or more consecutive spikes occurring without an interdischarge interval, with a total duration of less than 0.5 s.[16] Sharp waves are similar to spikes but have a longer duration, lasting between 70 and 200 ms.
Recent reviews indicate that different types of malformations of cortical development (MCDs) can exhibit similar interictal and seizure-onset EEG patterns, reflecting shared underlying biological mechanisms. However, certain EEG patterns appear to be specific to particular MCD types, suggesting differences in their neuronal networks.
Most interictal EEG patterns are observed in both scalp and intracranial recordings, while rhythmic epileptiform discharge types 1 and 2 are found exclusively on scalp EEG. In focal cortical dysplasia (FCD), only repetitive bursting spikes and sporadic spikes have been identified on intracranial EEG. Seizure-onset patterns are also largely detected in both scalp and intracranial EEGs, though some patterns are unique to specific conditions. For instance, suppression on scalp EEG and δ brush on intracranial EEG are associated with FCD, while focal fast waves on scalp EEG are linked to tuberous sclerosis complex (TSC).[17] The interictal epileptiform discharges that may be observed are explained below.
3-Hz spike-and-wave patterns
Spike-and-wave discharges (SWDs) are the primary diagnostic criterion for childhood absence epilepsy (CAE) and serve as the electrographic hallmark of absence seizures. Although characteristic of absence seizures, these discharges can also occur in other types of generalized seizures.
The waking background EEG activity remains normal. SWDs present as bisynchronous, symmetric discharges with sudden onset and resolution. The frequency of these signals ranges from 3.5 to 4 Hz at onset and slows to 2.5 to 3 Hz at resolution. The greatest amplitude occurs at the superior frontal electrodes. EEG discharges are reactive, inhibited by eye-opening and alertness, and readily activated by hyperventilation and hypoglycemia. While traditionally considered subclinical, response testing may reveal subtle declines in maximal alertness.[18]
SWDs result from thalamocortical oscillations, the same mechanism underlying sleep spindles.[19] Recent studies describe alternating spikes and waves as a progression of neuronal excitation during the spike component, followed by postexcitatory silence during the wave component. Analyses of layer-specific activity suggest that thalamic inputs drive a sequence of cellular-synaptic events essential for spike generation, while intracortical oscillations produce the wave component. These findings align with previous research, which proposed that oscillatory cortical waves provide adequate time windows for integrating thalamocortical inputs and feedback responses during seizures.[20]
Centrotemporal (Rolandic) spikes
Centrotemporal spikes, also known as Rolandic spikes, are characteristic of benign focal epilepsy of childhood with centrotemporal spikes (BECTS). These epileptic spikes commonly exhibit horizontal dipoles, with maximal negativity in the centrotemporal area and positivity in the frontal area. EEG discharges may be unilateral or bilateral, or they may shift laterality. The signals often appear asynchronously between hemispheres.
Recent studies suggest that disruptions in the Rolandic thalamocortical circuit contribute to both an abundance of epileptiform spikes and a reduction in sleep spindles—physiological thalamocortical rhythms associated with sleep-dependent learning. In the Rolandic cortex, these disturbances manifest during nonrapid eye movement (NREM) sleep, with epileptiform activity linked to seizures and a reduction in sleep spindles associated with cognitive symptoms.[21][22]
EEG discharges are not affected by hyperventilation or photic stimulation but are activated by drowsiness and sleep.[23] More than 1 seizure focus may be present, and the spike location may shift toward or away from the Rolandic area, which corresponds to the central sulcus, also known as the centrotemporal region.[24][25] Seizures are typically brief focal events but may secondarily generalize into tonic-clonic seizures. Seizures occur predominantly during sleep and are infrequent during wakefulness.
Epileptic encephalopathy with continuous spike-and-wave during sleep
Continuous spike-and-wave activity during sleep is observed in various seizure subtypes and epilepsy syndromes. Structural brain abnormalities, genetic mutations, and metabolic derangements can contribute to this occurrence.[26] Recent investigations have led to the reclassification of this pattern as epileptic encephalopathy with spike-and-wave activation in sleep (EE-SWAS). This pattern is associated with cognitive decline and language impairment, particularly in children. EEG analysis may provide prognostic insights, as epileptiform activity in EE-SWAS has been correlated with language outcomes at 1-year follow-up.[27][28][29]
Slow spike-and-wave discharges
These bilaterally synchronous discharges characterize symptomatic generalized epilepsies and serve as the hallmark EEG feature of Lennox-Gastaut syndrome (LGS), a severe childhood-onset developmental and epileptic encephalopathy (DEE).[30] These discharges typically occur at a frequency of 1 to 2.5 Hz. Slow spike-and-wave patterns may emerge from a previously normal EEG or evolve from hypsarrhythmia, as seen in infantile epileptic spasms syndrome, or multiple independent sharp-wave foci. The waking EEG background demonstrates generalized slowing, and during sleep, these discharges may increase, leading to electrical status epilepticus during sleep (ESES).[31] The spikes are most prominent in the frontal and temporal regions.[32]
Polyspike-and-wave bursts
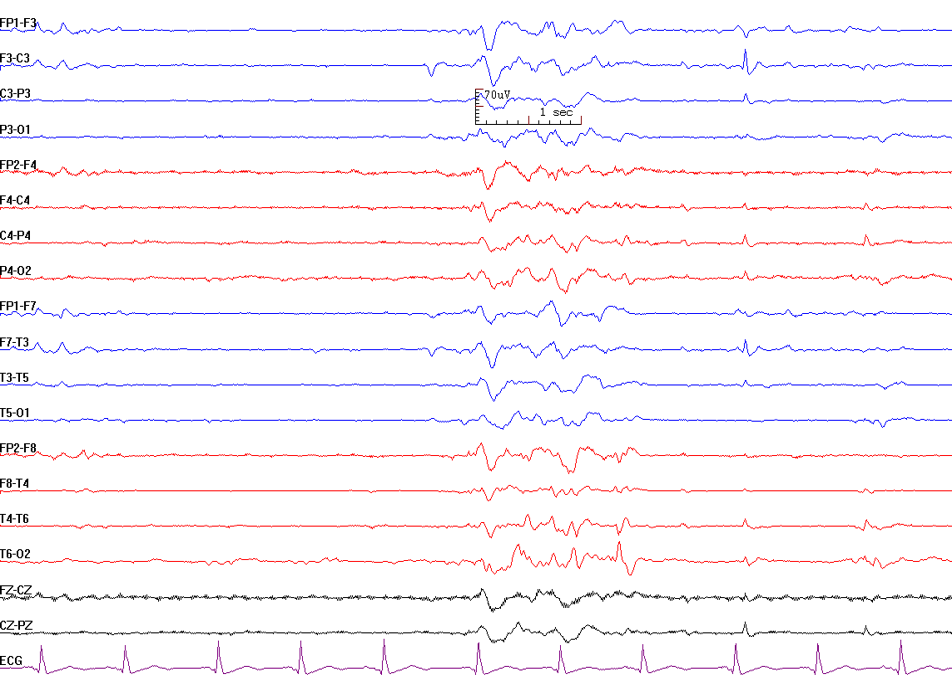
This complex of repetitive spikes followed by a wave component occurs in generalized epilepsy and, less frequently, in focal epilepsy (see Image. Polyspike-and-Wave). Generalized polyspike-and-wave discharges are common in myoclonic epilepsy. Myoclonus and bursts of generalized spike or polyspike slow-wave discharges with high amplitude often precede seizure onset. These manifestations are rapidly followed by tonic seizures featuring generalized fast activity at 10 Hz or higher. This underrecognized seizure type has been termed a myoclonic-to-tonic seizure.[33]
Examples of myoclonic epilepsy include juvenile myoclonic epilepsy and progressive myoclonic epilepsy. Polyspike-and-wave discharges typically occur at 3.5 to 5 Hz, referred to as "fast spike-and-wave," and exhibit a generalized frontocentral predominance with bursts at 1 to 2 Hz.[34] Myoclonic epilepsy primarily affects the upper extremities but can also involve the lower extremities. Recent case reports have described irregular slow-wave activity combined with generalized polyspike-and-wave discharges triggered by food intake, progressing to bursts of generalized polyspike-wave complexes.[35] Photic stimulation frequently activates these discharges.
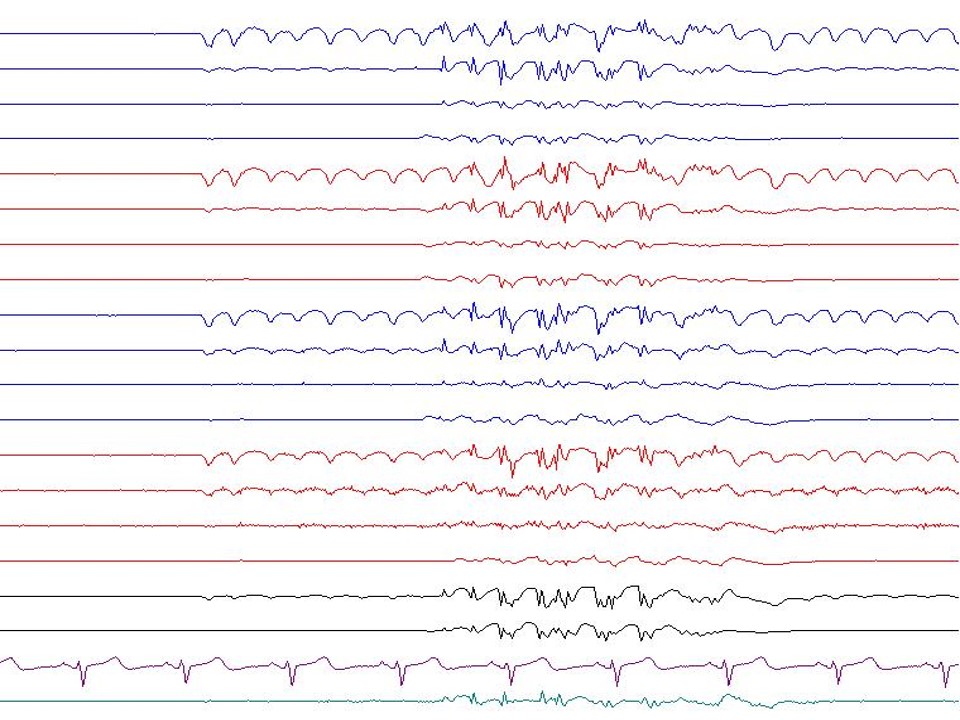
Generalized spike-and-wave patterns
A single spike is followed by a wave component in these discharges, which are commonly observed in primary generalized epilepsy (see Image. Generalized Spike-and-Wave). Generalized spike-and-wave (GSW) discharges are the hallmark EEG feature of idiopathic generalized epilepsies.[36] When present in idiopathic generalized epilepsy, GSWs occur against a normal background without other epileptiform abnormalities.
Lateralized periodic discharges
Lateralized periodic discharges (LPDs), also known as periodic lateralized epileptiform discharges (PLEDs), consist of repetitive focal discharges occurring at regular intervals. LPDs are associated with acute focal structural lesions and can also emerge following the resolution of partial-onset status epilepticus.[37] These discharges do not have a defined morphology and may present as spikes, sharps, polyspikes, or polyspike-and-wave patterns. Temporal LPDs are classically described in herpes simplex encephalitis, though LPDs can also arise from other brain infections, tumors, Creutzfeldt-Jakob disease, and acute brain injuries such as subarachnoid hemorrhage, stroke, or traumatic brain injury.[38]
Recent reviews have proposed a subtype known as "LPDs-max," characterized by spiky or sharp LPDs followed by slow after-waves or periods of flattening, producing a triphasic morphology. This pattern has been linked to focal nonconvulsive status epilepticus and is sometimes associated with subtle motor signs and epileptic seizures. LPDs-max manifests as periodic polyspike-wave activity or focal burst-suppression-like patterns. This subtype predominantly affects the temporoparietooccipital regions and is often refractory to antiseizure medications.[39]
Bilateral independent periodic discharges
Bilateral independent periodic discharges (BIPDs), also known as bilateral periodic lateralized epileptiform discharges (BiPLEDs), are lateralized periodic discharges (LPDs) that originate from 2 locations, each from a different cerebral hemisphere. Advanced Gerstmann-Sträussler-Scheinker syndrome (GSS) has shown BIPDs in both temporal areas.[40] The 2 LPDs are independent, not synchronous, and may occur at different frequencies. A recent observational study found an association between BIPDs, mortality, and unfavorable outcomes among critically ill children.[41]
Generalized periodic discharges
Generalized periodic discharges (GPDs) are synchronous, repetitive discharges occurring at regular intervals. The interdischarge intervals are usually quantifiable, and each discharge maintains a similar morphology. These patterns are observed in multiple conditions, including anoxic brain injury, hypothermia, status epilepticus (during or after resolution), and infectious, toxic, or metabolic encephalopathy.[42] Disruption of thalamocortical pathways underlies GPD generation.[43] Prognosis is often guarded and ultimately depends on the underlying etiology. GPDs may be associated with nonconvulsive status epilepticus but do not inherently represent status epilepticus. Recent findings described a rare case of GPDs, generally referred to as periodic sharp-wave complexes, during the very early stages of Creutzfeldt-Jakob disease.[44]
Subclinical electroencephalographic discharges of adults
Subclinical rhythmic EEG discharges in adults (SREDA) represent a rare and benign EEG variant generally considered epileptiform. This pattern consists of sharply contoured rhythmic θ activity bilaterally, with maximal activity in the parietal or posterior head region. These paroxysms are not associated with any objective or subjective clinical manifestations and are generally not considered epileptiform but may resemble epileptiform activity.[45] SREDA is typically observed in adulthood, though a few reports have documented occurrences in children.[46]
Subclinical epileptiform discharges (SED) in individuals with Alzheimer disease have been linked to accelerated cognitive decline, with a strong association between decline severity and the frequency and spatial distribution of spikes, particularly in the left temporal region. These findings indicate that SED may contribute to disease progression and represents a potential therapeutic target.[47][48]
A rarely observed EEG pattern, sometimes classified as a benign variant but generally considered epileptiform, has been documented in children. This pattern can resemble an electrographic seizure due to the sudden evolution of high-voltage generalized fast (5 to 6 Hz) spike-and-wave activity, often occurring in a recurrent pattern.[49]
Brief (potentially ictal) rhythmic epileptiform discharges
Brief, potentially ictal rhythmic epileptiform discharges [B(i)RDs] are rare EEG patterns primarily observed in critically ill patients and neonates. Sudden runs of sharply contoured θ activity lasting up to 3 seconds characterize these discharges. These patterns, also referred to as "brief electrographic rhythmic discharges" (BERDs), are associated with epileptogenic foci in refractory epilepsy and cerebral injury in critically ill patients.[50] However, current literature indicates an increased seizure risk in both critically ill and noncritically ill adults. In critically ill patients, B(i)RDs correlate with acute brain injury and worse functional outcomes. Additionally, these discharges serve as significant risk factors for continuous EEG seizures in critically ill patients.
Among noncritically ill adults, B(i)RDs are linked to epilepsy, with a higher likelihood of drug resistance. The location of these discharges provides some predictive value for the seizure onset zone compared to other interictal epileptiform discharges. Paroxysmal fast activity (PFA) falls under the classification of B(i)RDs, with similar clinical significance regardless of exact frequency cutoffs. Studies have also proposed B(i)RDs as a biomarker for seizure activity and seizure onset zone localization. In patients with status epilepticus, resolution or reduction of B(i)RDs often coincides with seizure resolution.[51][52][53]
Nonepileptiform Abnormalities
Nonepileptiform abnormalities on EEG often manifest as slowing, which reflects cerebral dysfunction rather than epileptic activity. The characteristics and distribution of slowing help differentiate between diffuse and focal brain pathology. Based on waveform morphology, slowing may be classified as polymorphic, which has variable shapes, or rhythmic, which has consistent frequency. Polymorphic slowing is typically associated with structural dysfunction, whereas rhythmic slowing may suggest an epileptiform process.
Slowing may also be diffuse or focal, depending on the extent and location of cerebral involvement.[54] Diffuse slowing reflects global cerebral dysfunction. Frequencies fall within the θ or δ ranges and may present with high or low amplitude. Etiologies include sedative medications, metabolic or toxic encephalopathy, cerebral infections such as meningoencephalitis, and deep midline brainstem structural lesions.
Focal slowing reflects focal cerebral dysfunction and may be continuous or intermittent. Continuous focal slowing often indicates structural abnormalities and is observed in conditions such as brain tumors, stroke, traumatic brain injury, and intracerebral hemorrhage. Intermittent focal slowing varies based on its location and may present as frontal, occipital, or temporal intermittent rhythmic δ activity, as explained below.
Frontal intermittent rhythmic δ activity (FIRDA) appears as a rhythmic slow-wave pattern lasting several seconds over the anterior EEG leads. FIRDA occurs in individuals without structural brain lesions but with metabolic encephalopathy, neurodegenerative disease, hypoxic encephalopathy due to cardiac arrest, systemic infection, or encephalitis.[55]
Occipital intermittent rhythmic δ activity (OIRDA) presents as symmetric sinusoidal occipital-maximal activity and is frequently associated with childhood occipital epilepsy. OIRDA without a prominent field effect may also be lateralized or maximal on the hemispheric side ipsilateral to more definitive epileptiform discharges in focal epilepsies. A notched morphology often results from intermixed sharp wave activity, though these sharp waves do not occur repetitively. OIRDA is suggested to originate cortically and is associated with idiopathic epilepsies rather than the subcortical generators hypothesized in primary generalized absence epilepsy.[56]
Temporal intermittent rhythmic δ activity (TIRDA) is linked to interictal epileptiform discharges from the temporal regions. FIRDA, OIRDA, and TIRDA are all patterns associated with epilepsy.[57]
Other Diffuse or Focal Abnormal Electroencephalogram Patterns
EEG plays a critical role in detecting cortical ischemia, identifying seizures, and predicting neurological abnormalities. One useful pattern is electrocerebral inactivity (ECI), which refers to the absence of detectable EEG activity at a sensitivity of 2 µV. ECI may also serve as a reference for intraoperative cerebral protection.[58] This finding may support a brain death diagnosis but is not specific to this condition, as it can also occur with deep sedation, severe hypothermia, and certain metabolic disorders. Specific criteria must be met when ECI is used for brain death determination, including a 30-minute high-quality EEG recording with a full set of scalp electrodes, interelectrode impedances between 100 to 10,000 Ω, and an interelectrode distance of at least 10 cm.[59]
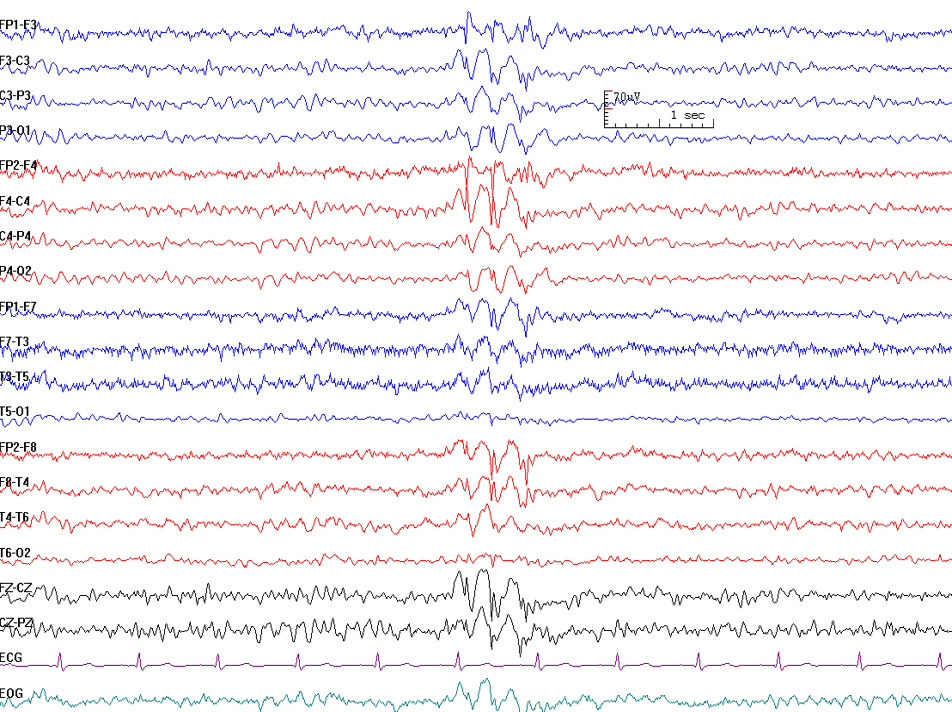
Burst suppression pattern (BSP) was first described by Derbyshire et al. in 1936 in relation to the anesthetic effects of tribromoethanol, with the term later coined by Swank and Watson in 1949. BSP is characteristic of neonatal and infantile-onset developmental and epileptic encephalopathies (DEEs), conditions associated with high early-life mortality.[60] This pattern may also be secondary to hypothermia, metabolic disorders, general anesthesia, or anoxic brain injury from cardiac arrest.
BSP can also serve as a marker of cerebral damage and is sometimes used as a therapeutic endpoint in refractory status epilepticus or refractory intracranial hypertension. The goal in these cases is to limit bursts to 1 per EEG page or fewer. In anoxic brain injury, bursts may be accompanied by myoclonic jerks.[61][62][63] BSP has also been implicated in postoperative delirium and negative outcomes such as frailty and intraoperative hypotension (IOH).[64] The pattern consists of alternating high-voltage slow waves (bursts) and periods of low voltage or isoelectricity (suppression), reflecting cortical hyperexcitability due to compromised inhibition (see Image. Burst Suppression Pattern).
Beyond intensive care and operating room settings, BSP is extremely rare.[65][66] Further deepening of coma from burst suppression results in severe low-amplitude slowing without reactivity, producing an EEG that appears relatively flat.[67]
Breach rhythm, also known as the breach effect, is a focal, asymmetric pattern characterized by high-voltage, arch-like waveforms that sometimes exhibit a spiky morphology. This rhythm may present as an irregular pattern, occasionally with sharp activity resembling epileptiform discharges. Typically associated with focal skull abnormalities such as craniotomy, breach rhythm results from decreased impedance, allowing enhanced signal detection from the cortex in areas where bone or tissue is absent.
The pattern is most prominent over central and temporal regions, displaying sharp contours with a frequency range of 6 to 11 Hz, though variations with faster or slower wave activity can occur. Serial trains of breach rhythm are easily recognized, while isolated spike-like or sharp-contoured waveforms are more likely to be mistaken for epileptiform activity. Unlike true epileptiform discharges, breach rhythm lacks aftercoming slow waves and does not propagate to other areas. If associated with polymorphic δ activity, underlying brain damage should be considered. When present over the central region, breach rhythm can be blocked by voluntary movement due to the presence of normal underlying μ activity. In some cases, this rhythm persists during sleep and appears as increased voltage along sleep spindles in stage 2 sleep.
Clinical Significance
Understanding abnormal EEG waveforms and distinguishing them from normal variations is essential. A normal EEG does not exclude epilepsy, as EEG sensitivity for epilepsy detection is less than 50%. Additionally, interictal discharges and other EEG abnormalities may be present in healthy individuals. Unnecessary EEG testing can lead to misdiagnoses and inappropriate treatments if not interpreted properly.
Breach rhythm, a normal variant seen with skull defects, may exhibit focal, sharply contoured morphology.[68] Several EEG characteristics differentiate breach rhythm from epileptiform abnormalities, but clinical correlation with a history of craniotomy or magnetic resonance imaging findings of a skull defect improves diagnostic accuracy. EEG abnormalities should always be interpreted within the clinical context.
Scalp EEG, particularly IEDs, plays a crucial role in predicting surgical outcomes. A nomogram developed for seizure freedom prediction provides individualized postoperative assessments and offers valuable insights into the association between preoperative findings and outcomes.[69]
Enhancing Healthcare Team Outcomes
An interprofessional team involving EEG technicians, nurses, and physicians enhances the care of individuals with abnormal EEG findings. Educating healthcare professionals on EEG interpretation and abnormal waveforms is essential for accurate diagnosis and management. Proper training in EEG report analysis supports clinical decision-making and improves patient outcomes.
A recent study highlighted the role of abnormal EEG findings in assessing seizure risk in hospitalized patients alongside factors such as age, focal seizures, and low protein levels. EEG may play a crucial role in inpatient evaluation by guiding clinical management and supporting a comprehensive approach to patient care.[70]
Media
(Click Image to Enlarge)
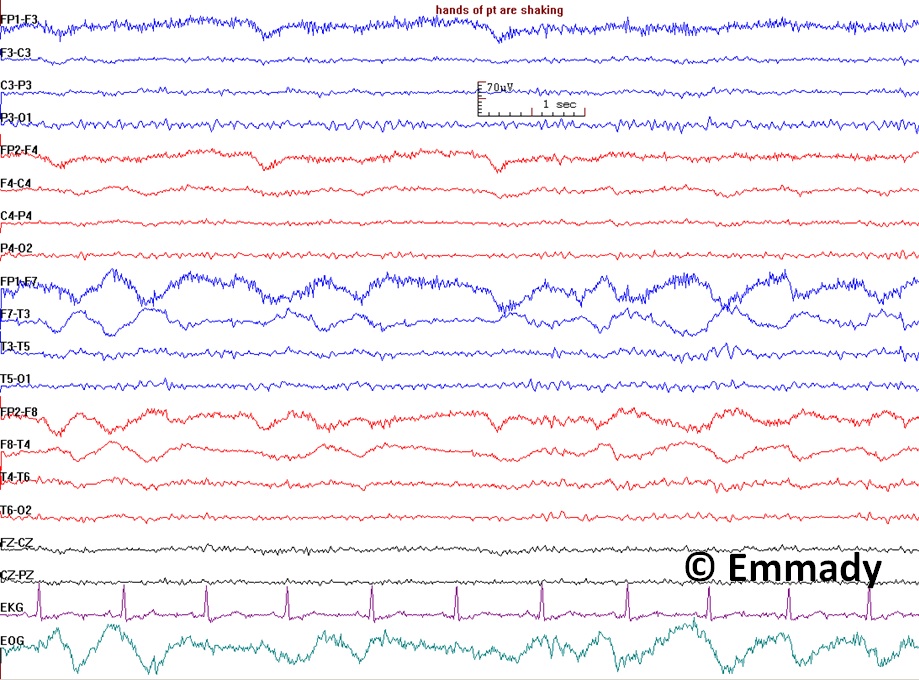
Electroencephalogram Pattern for a Nonepileptic Seizure. This electroencephalogram recording demonstrates features associated with nonepileptic seizures, including handshaking and lateral eye movements. The absence of epileptiform discharges on the EEG, coupled with these physiological movements, suggests a nonepileptic event rather than a seizure originating from epileptic activity. Clinical correlation is essential for correct diagnosis and appropriate management.
Contributed by Prabhu Emmady, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Syamsundararao T, Selvarani A, Rathi R, Vini Antony Grace N, Selvaraj D, Almutairi KMA, Alonazi WB, Priyan KSA, Mosissa R. An Efficient Signal Processing Algorithm for Detecting Abnormalities in EEG Signal Using CNN. Contrast media & molecular imaging. 2022:2022():1502934. doi: 10.1155/2022/1502934. Epub 2022 Sep 21 [PubMed PMID: 36213561]
Kadian R, Vemireddy LP, Lui F, Kumar A. Breach Rhythm. StatPearls. 2025 Jan:(): [PubMed PMID: 30480975]
Duvuru S, Sanker V, Mishra RK, Sharma AK, Lim SL, Baskar N, Sharma VK. Ancillary tests for brain death. Frontiers in neurology. 2024:15():1491263. doi: 10.3389/fneur.2024.1491263. Epub 2025 Jan 7 [PubMed PMID: 39839883]
Koshiyama D, Miyakoshi M, Tanaka-Koshiyama K, Joshi YB, Sprock J, Braff DL, Light GA. Abnormal phase discontinuity of alpha- and theta-frequency oscillations in schizophrenia. Schizophrenia research. 2021 May:231():73-81. doi: 10.1016/j.schres.2021.03.007. Epub 2021 Mar 27 [PubMed PMID: 33780847]
Clemens B, Puskás S, Dömötör J. [EEG abnormalities indicating the genetic determination of epilepsies]. Ideggyogyaszati szemle. 2022 Sep 30:75(9-10):295-305. doi: 10.18071/isz.75.0295. Epub [PubMed PMID: 36218119]
Brigo F, Storti M. Triphasic waves. American journal of electroneurodiagnostic technology. 2011 Mar:51(1):16-25 [PubMed PMID: 21516927]
Van Zandycke M, Orban LC, Vander Eecken HV. [Occurrence of triphasic waves in two cases of thyrotoxic crisis (author's transl)]. Acta neurologica Belgica. 1977 Mar-Apr:77(2):115-20 [PubMed PMID: 868471]
Level 3 (low-level) evidenceBICKFORD RG, BUTT HR. Hepatic coma: the electroencephalographic pattern. The Journal of clinical investigation. 1955 Jun:34(6):790-9 [PubMed PMID: 14381508]
Foreman B. Can We Distinguish Triphasic Waves From Other Generalized Periodic Discharges? Do We Need to? Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2021 Sep 1:38(5):362-365. doi: 10.1097/WNP.0000000000000765. Epub [PubMed PMID: 34155184]
Emmady PD, Murr NI. EEG Triphasic Waves. StatPearls. 2024 Jan:(): [PubMed PMID: 32491611]
Kotchetkov IS, Freund B, Husari K, Kaplan PW. In the Kingdom of Triphasic Waves, White Matter Is the Eminence Grise. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2021 Nov 1:38(6):547-552. doi: 10.1097/WNP.0000000000000721. Epub [PubMed PMID: 32941293]
Zacharaki EI, Mporas I, Garganis K, Megalooikonomou V. Spike pattern recognition by supervised classification in low dimensional embedding space. Brain informatics. 2016 Jun:3(2):73-83 [PubMed PMID: 27747608]
Wirrell EC. Prognostic significance of interictal epileptiform discharges in newly diagnosed seizure disorders. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010 Aug:27(4):239-48. doi: 10.1097/WNP.0b013e3181ea4288. Epub [PubMed PMID: 20634717]
So EL. Interictal epileptiform discharges in persons without a history of seizures: what do they mean? Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010 Aug:27(4):229-38. doi: 10.1097/WNP.0b013e3181ea42a4. Epub [PubMed PMID: 20634716]
Destexhe A. Spike-and-wave oscillations based on the properties of GABAB receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998 Nov 1:18(21):9099-111 [PubMed PMID: 9787013]
Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 Version. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2021 Jan 1:38(1):1-29. doi: 10.1097/WNP.0000000000000806. Epub [PubMed PMID: 33475321]
Shakhatreh L, Janmohamed M, Baker AA, Willard A, Laing J, Rychkova M, Chen Z, Kwan P, O'Brien TJ, Perucca P. Interictal and seizure-onset EEG patterns in malformations of cortical development: A systematic review. Neurobiology of disease. 2022 Nov:174():105863. doi: 10.1016/j.nbd.2022.105863. Epub 2022 Sep 19 [PubMed PMID: 36165814]
Level 1 (high-level) evidenceAlbuja AC, Ighodaro ET, Khan GQ. Absence Seizure. StatPearls. 2025 Jan:(): [PubMed PMID: 29763042]
Avoli M. A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia. 2012 May:53(5):779-89. doi: 10.1111/j.1528-1167.2012.03421.x. Epub 2012 Feb 23 [PubMed PMID: 22360294]
Level 3 (low-level) evidenceTerlau J, Yang JW, Khastkhodaei Z, Seidenbecher T, Luhmann HJ, Pape HC, Lüttjohann A. Spike-wave discharges in absence epilepsy: segregation of electrographic components reveals distinct pathways of seizure activity. The Journal of physiology. 2020 Jun:598(12):2397-2414. doi: 10.1113/JP279483. Epub 2020 Apr 30 [PubMed PMID: 32144956]
Li Q, Westover MB, Zhang R, Chu CJ. Computational Evidence for a Competitive Thalamocortical Model of Spikes and Spindle Activity in Rolandic Epilepsy. Frontiers in computational neuroscience. 2021:15():680549. doi: 10.3389/fncom.2021.680549. Epub 2021 Jun 18 [PubMed PMID: 34220477]
Spencer ER, Chinappen D, Emerton BC, Morgan AK, Hämäläinen MS, Manoach DS, Eden UT, Kramer MA, Chu CJ. Source EEG reveals that Rolandic epilepsy is a regional epileptic encephalopathy. NeuroImage. Clinical. 2022:33():102956. doi: 10.1016/j.nicl.2022.102956. Epub 2022 Feb 7 [PubMed PMID: 35151039]
Lee YJ, Hwang SK, Kwon S. The Clinical Spectrum of Benign Epilepsy with Centro-Temporal Spikes: a Challenge in Categorization and Predictability. Journal of epilepsy research. 2017 Jun:7(1):1-6. doi: 10.14581/jer.17001. Epub 2017 Jun 30 [PubMed PMID: 28775948]
Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia. 1998:39 Suppl 4():S32-41 [PubMed PMID: 9637591]
Amrutkar CV, Riel-Romero RM. Rolandic Epilepsy Seizure. StatPearls. 2025 Jan:(): [PubMed PMID: 30521266]
Singhal NS, Sullivan JE. Continuous Spike-Wave during Slow Wave Sleep and Related Conditions. ISRN neurology. 2014:2014():619079. doi: 10.1155/2014/619079. Epub 2014 Jan 30 [PubMed PMID: 24634784]
Sager G, Takis G, Vatansever Pinar Z, Duzkalir H, Turkyilmaz A, Çağ Y, Akin Y. Evaluation of long-term neurocognitive functions in patients with epileptic encephalopathy with continuous spike-and-wave during sleep (CSWS)/epileptic encephalopathy with spike-and-wave activation in sleep (EE-SWAS). Neurophysiologie clinique = Clinical neurophysiology. 2023 Feb:53(1):102861. doi: 10.1016/j.neucli.2023.102861. Epub 2023 Apr 12 [PubMed PMID: 37058916]
Saraf UU, Asranna A, Menon RN, Mohan P M, Vp V, Radhakrishnan A, Cherian A, V Thomas S. Predictors of one-year language and seizure outcomes in children with epileptic encephalopathy with continuous spike-and-wave during sleep (CSWS). Seizure. 2020 Oct:81():315-324. doi: 10.1016/j.seizure.2020.08.025. Epub 2020 Aug 28 [PubMed PMID: 32961503]
Azeem A, Kirton A, Appendino JP, Kozlik S, Mineyko A. Automated quantification of spike-wave activity may be used to predict the development of electrical status epilepticus in sleep (ESES) in children with perinatal stroke. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2021 Jan:132(1):146-153. doi: 10.1016/j.clinph.2020.11.003. Epub 2020 Nov 13 [PubMed PMID: 33278667]
Strzelczyk A, Schubert-Bast S. Expanding the Treatment Landscape for Lennox-Gastaut Syndrome: Current and Future Strategies. CNS drugs. 2021 Jan:35(1):61-83. doi: 10.1007/s40263-020-00784-8. Epub 2021 Jan 21 [PubMed PMID: 33479851]
Ikeda A, Yamamoto A, Ichikawa K, Tsuyusaki Y, Tsuji M, Iai M, Enomoto Y, Murakami H, Kurosawa K, Miyatake S, Matsumoto N, Goto T. Epilepsy in Christianson syndrome: Two cases of Lennox-Gastaut syndrome and a review of literature. Epilepsy & behavior reports. 2020:13():100349. doi: 10.1016/j.ebr.2019.100349. Epub 2019 Dec 5 [PubMed PMID: 31879735]
Markand ON. Slow spike-wave activity in EEG and associated clinical features: often called 'Lennox' or "Lennox-Gastaut' syndrome. Neurology. 1977 Aug:27(8):746-57 [PubMed PMID: 407485]
Zhou Z, Gong P, Jiao X, Niu Y, Xu Z, Qin J, Yang Z. A generalized seizure type: Myoclonic-to-tonic seizure. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2024 Aug:164():24-29. doi: 10.1016/j.clinph.2024.04.011. Epub 2024 Apr 23 [PubMed PMID: 38823261]
Level 2 (mid-level) evidenceZhang MW, Bustros ST, Gaston TE, Descartes M, Agnihotri SP. Short Report: Clinical Features and Epilepsy Monitoring in an Adult With 22q11.2 Deletion Syndrome. The Neurohospitalist. 2024 Jul:14(3):273-277. doi: 10.1177/19418744241228618. Epub 2024 Jan 17 [PubMed PMID: 38895014]
Wang X, Chen B, Jin L, Zhang W, Liu Y. Eight years follow-up of a generalized epilepsy patient with eating-induced late-onset epileptic spasms and atypical absence with myoclonic jerks. Brain & development. 2021 Jan:43(1):160-165. doi: 10.1016/j.braindev.2020.07.018. Epub 2020 Aug 10 [PubMed PMID: 32792174]
Asadi-Pooya AA, Farazdaghi M. Generalized spike-waves in idiopathic generalized epilepsies: Does their frequency matter? Brain and behavior. 2024 Oct:14(10):e70023. doi: 10.1002/brb3.70023. Epub [PubMed PMID: 39363786]
Lin L, Drislane FW. Lateralized Periodic Discharges: A Literature Review. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 May:35(3):189-198. doi: 10.1097/WNP.0000000000000448. Epub [PubMed PMID: 29718828]
García-Morales I, García MT, Galán-Dávila L, Gómez-Escalonilla C, Saiz-Díaz R, Martínez-Salio A, de la Peña P, Tejerina JA. Periodic lateralized epileptiform discharges: etiology, clinical aspects, seizures, and evolution in 130 patients. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2002 Apr:19(2):172-7 [PubMed PMID: 11997729]
Level 2 (mid-level) evidenceGelisse P, Crespel A, Genton P, Jallon P, Kaplan PW. Lateralized Periodic Discharges: Which patterns are interictal, ictal, or peri-ictal? Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2021 Jul:132(7):1593-1603. doi: 10.1016/j.clinph.2021.04.003. Epub 2021 Apr 27 [PubMed PMID: 34034086]
Yazawa S, Tsuruta K, Sugimoto A, Suzuki Y, Yagi K, Matsuhashi M, Yoshimura M, Takashima H. Appearance of bitemporal periodic EEG activity in the last stage of Gerstmann-Sträussler-Scheinker syndrome (Pro102Leu): A case report. Clinical neurology and neurosurgery. 2021 May:204():106602. doi: 10.1016/j.clineuro.2021.106602. Epub 2021 Mar 20 [PubMed PMID: 33774505]
Level 3 (low-level) evidenceFung FW, Parikh DS, Massey SL, Fitzgerald MP, Vala L, Donnelly M, Jacobwitz M, Kessler SK, Xiao R, Topjian AA, Abend NS. Periodic Discharges in Critically Ill Children: Predictors and Outcome. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2024 May 1:41(4):297-304. doi: 10.1097/WNP.0000000000000986. Epub 2023 Dec 1 [PubMed PMID: 38079254]
Sully KE, Husain AM. Generalized Periodic Discharges: A Topical Review. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 May:35(3):199-207. doi: 10.1097/WNP.0000000000000460. Epub [PubMed PMID: 29718829]
Foreman B, Claassen J, Abou Khaled K, Jirsch J, Alschuler DM, Wittman J, Emerson RG, Hirsch LJ. Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012 Nov 6:79(19):1951-60. doi: 10.1212/WNL.0b013e3182735cd7. Epub 2012 Oct 3 [PubMed PMID: 23035068]
Level 2 (mid-level) evidenceMatsubayashi T, Akaza M, Hayashi Y, Hamaguchi T, Satoh K, Kosami K, Ae R, Kitamoto T, Yamada M, Shimohata T, Yokota T, Sanjo N. Specific electroencephalogram features in the very early phases of sporadic Creutzfeldt-Jakob disease. Journal of the neurological sciences. 2022 Jun 15:437():120265. doi: 10.1016/j.jns.2022.120265. Epub 2022 Apr 18 [PubMed PMID: 35472604]
Shaji SA, E G A, Bahuleyan B, Noushad F, Vincent SJ, Suresh A, Radhakrishnan A. SREDA: An Uncommon and Misleading EEG Rhythm. The Neurodiagnostic journal. 2023 Dec:63(4):245-251. doi: 10.1080/21646821.2023.2249773. Epub 2023 Dec 13 [PubMed PMID: 37819725]
Bosisio L, Mancardi MM, Boeri S, Nobili L, Nobile G, Siri L, Prato G, Canale E. Subclinical rhythmic EEG discharge of adults (SREDA) in pediatric population: A case series with systematic review of the literature. Epileptic disorders : international epilepsy journal with videotape. 2025 Feb:27(1):71-81. doi: 10.1002/epd2.20294. Epub 2024 Oct 16 [PubMed PMID: 39412218]
Level 1 (high-level) evidenceHorvath AA, Papp A, Zsuffa J, Szucs A, Luckl J, Radai F, Nagy F, Hidasi Z, Csukly G, Barcs G, Kamondi A. Subclinical epileptiform activity accelerates the progression of Alzheimer's disease: A long-term EEG study. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2021 Aug:132(8):1982-1989. doi: 10.1016/j.clinph.2021.03.050. Epub 2021 May 8 [PubMed PMID: 34034963]
Yeh WC, Hsu CY, Li KY, Chien CF, Huang LC, Yang YH. Association between Subclinical Epileptiform Discharge and the Severity of Cognitive Decline in Alzheimer's Disease: A Longitudinal Cohort Study. Journal of Alzheimer's disease : JAD. 2022:90(1):305-312. doi: 10.3233/JAD-220567. Epub [PubMed PMID: 36120783]
Goeden M, Bansal LR. Subclinical Rhythmic EEG Discharge of Adult (SREDA) in a Child With Generalized Epilepsy and Literature Review of SREDA in Children. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 May:35(3):270-272. doi: 10.1097/WNP.0000000000000408. Epub [PubMed PMID: 28800038]
Yoo JY, Rampal N, Petroff OA, Hirsch LJ, Gaspard N. Brief potentially ictal rhythmic discharges in critically ill adults. JAMA neurology. 2014 Apr:71(4):454-62. doi: 10.1001/jamaneurol.2013.6238. Epub [PubMed PMID: 24535702]
Level 2 (mid-level) evidenceYoo JY. BIRDs (Brief Potentially Ictal Rhythmic Discharges) watching during EEG monitoring. Frontiers in neurology. 2022:13():966480. doi: 10.3389/fneur.2022.966480. Epub 2022 Aug 23 [PubMed PMID: 36081872]
Yoo JY, Jetté N, Kwon CS, Young J, Marcuse LV, Fields MC, Gaspard N, Hirsch LJ. Brief potentially ictal rhythmic discharges and paroxysmal fast activity as scalp electroencephalographic biomarkers of seizure activity and seizure onset zone. Epilepsia. 2021 Mar:62(3):742-751. doi: 10.1111/epi.16822. Epub 2021 Feb 12 [PubMed PMID: 33576500]
Naves PVF, Caboclo LO. Independent risk factors for seizures in critically ill patients on continuous EEG. Epileptic disorders : international epilepsy journal with videotape. 2022 Apr 1:24(2):287-294. doi: 10.1684/epd.2021.1388. Epub [PubMed PMID: 34825889]
St. Louis EK, Frey LC, Britton JW, Frey LC, Hopp JL, Korb P, Koubeissi MZ, Lievens WE, Pestana-Knight EM, St. Louis EK. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants. 2016:(): [PubMed PMID: 27748095]
Kim KT, Roh YN, Cho NH, Jeon JC. Clinical Correlates of Frontal Intermittent Rhythmic Delta Activity Without Structural Brain Lesion. Clinical EEG and neuroscience. 2021 Jan:52(1):69-73. doi: 10.1177/1550059420922741. Epub 2020 May 15 [PubMed PMID: 32412802]
LaBarbera V, Nie D. Occipital intermittent rhythmic delta activity (OIRDA) in pediatric focal epilepsies: A case series. Epilepsy & behavior reports. 2021:16():100472. doi: 10.1016/j.ebr.2021.100472. Epub 2021 Jul 21 [PubMed PMID: 34401708]
Level 2 (mid-level) evidenceFreund BE, Mheir-Al-Saadi Z, Brinkmann BH, Tatum WO. "Temporal" intermittent rhythmic delta activity: the true localizing nature of TIRDA. Epileptic disorders : international epilepsy journal with videotape. 2022 Oct 1:24(5):947-951. doi: 10.1684/epd.2022.1459. Epub [PubMed PMID: 35816099]
McDevitt WM, Gul T, Jones TJ, Scholefield BR, Seri S, Drury NE. Perioperative electroencephalography in cardiac surgery with hypothermic circulatory arrest: a narrative review. Interactive cardiovascular and thoracic surgery. 2022 Sep 9:35(4):. doi: 10.1093/icvts/ivac198. Epub [PubMed PMID: 35904759]
Level 3 (low-level) evidenceSzurhaj W, Lamblin MD, Kaminska A, Sediri H, Société de Neurophysiologie Clinique de Langue Française. EEG guidelines in the diagnosis of brain death. Neurophysiologie clinique = Clinical neurophysiology. 2015 Mar:45(1):97-104. doi: 10.1016/j.neucli.2014.11.005. Epub 2015 Jan 14 [PubMed PMID: 25687591]
Yamamoto K, Baba S, Saito T, Nakagawa E, Sugai K, Iwasaki M, Fujita A, Fukuda H, Mizuguchi T, Kato M, Matsumoto N, Sasaki M. Synchronous heart rate reduction with suppression-burst pattern in KCNT1-related developmental and epileptic encephalopathies. Epilepsia open. 2023 Jun:8(2):651-658. doi: 10.1002/epi4.12705. Epub 2023 Feb 14 [PubMed PMID: 36740266]
Reeves AL, Westmoreland BF, Klass DW. Clinical accompaniments of the burst-suppression EEG pattern. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1997 Mar:14(2):150-3 [PubMed PMID: 9165410]
Level 3 (low-level) evidenceLobo FA, Vacas S, Rossetti AO, Robba C, Taccone FS. Does electroencephalographic burst suppression still play a role in the perioperative setting? Best practice & research. Clinical anaesthesiology. 2021 Jul:35(2):159-169. doi: 10.1016/j.bpa.2020.10.007. Epub 2020 Oct 31 [PubMed PMID: 34030801]
Jones KG, Lybbert C, Euler MJ, Huang J, Lunt S, Richards SV, Jessop JE, Larson A, Odell DH, Kuck K, Tadler SC, Mickey BJ. Diversity of electroencephalographic patterns during propofol-induced burst suppression. Frontiers in systems neuroscience. 2023:17():1172856. doi: 10.3389/fnsys.2023.1172856. Epub 2023 Jun 15 [PubMed PMID: 37397237]
Chen J, Li W, Chen Q, Zhou Z, Chen C, Hu Y, Si Y, Zou J. Optimizing anesthesia management based on early identification of electroencephalogram burst suppression risk in non-cardiac surgery patients: a visualized dynamic nomogram. Annals of medicine. 2024 Dec:56(1):2407067. doi: 10.1080/07853890.2024.2407067. Epub 2024 Sep 24 [PubMed PMID: 39317392]
Hawkes MA, Eliliwi M, Wijdicks EFM. The Origin of the Burst-Suppression Paradigm in Treatment of Status Epilepticus. Neurocritical care. 2024 Jun:40(3):849-854. doi: 10.1007/s12028-023-01877-0. Epub 2023 Nov 3 [PubMed PMID: 37921932]
Siddiqi AZ, Froese L, Gomez A, Sainbhi AS, Stein K, Park K, Vakitbilir N, Zeiler FA. The effect of burst suppression on cerebral blood flow and autoregulation: a scoping review of the human and animal literature. Frontiers in physiology. 2023:14():1204874. doi: 10.3389/fphys.2023.1204874. Epub 2023 Jun 7 [PubMed PMID: 37351255]
Level 2 (mid-level) evidenceNiedermeyer E, Sherman DL, Geocadin RJ, Hansen HC, Hanley DF. The burst-suppression electroencephalogram. Clinical EEG (electroencephalography). 1999 Jul:30(3):99-105 [PubMed PMID: 10578472]
Level 3 (low-level) evidenceBrigo F, Cicero R, Fiaschi A, Bongiovanni LG. The breach rhythm. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011 Nov:122(11):2116-20. doi: 10.1016/j.clinph.2011.07.024. Epub 2011 Aug 26 [PubMed PMID: 21872525]
Fitzgerald Z, Morita-Sherman M, Hogue O, Joseph B, Alvim MKM, Yasuda CL, Vegh D, Nair D, Burgess R, Bingaman W, Najm I, Kattan MW, Blumcke I, Worrell G, Brinkmann BH, Cendes F, Jehi L. Improving the prediction of epilepsy surgery outcomes using basic scalp EEG findings. Epilepsia. 2021 Oct:62(10):2439-2450. doi: 10.1111/epi.17024. Epub 2021 Aug 2 [PubMed PMID: 34338324]
Satar SNA, Mogan S, Jaafar WPN, Maghalingam S, Affendi FAR, Ng CF, Khoo CS, Chee YC, Hod R, Tan HJ. Characteristics of electroencephalogram changes and correlation with seizures in hospitalised patients. The Medical journal of Malaysia. 2023 Mar:78(2):149-154 [PubMed PMID: 36988523]