Introduction
Prenatal screening tests were first introduced in the 1970s with a single second-trimester serum test for maternal serum alpha-fetoprotein (MSAFP), which was used to detect neural tube defects. By the 1980s, maternal serum marker screening for aneuploidy became available. Since then, the range and complexity of prenatal screening options have continued to expand steadily.[1] Prenatal genetic screening evaluates the risk of a fetus having a specific genetic disorder, while prenatal diagnostic testing is used to confirm the presence of a genetic condition.[2] Prenatal diagnostic testing is typically performed through procedures such as chorionic villus sampling or amniocentesis.
Prenatal genetic screening was initially developed to detect trisomy 21 (T21, also known as Down syndrome) but has since expanded to include a wider range of genetic conditions, such as trisomy 13 (T13), trisomy 18 (T18), selected microdeletions, and sex chromosome abnormalities.[2] Screening options now include first-trimester screening, which combines nuchal translucency measurement via ultrasound with maternal serum analyte analysis, as well as second-trimester triple, quadruple, or penta screening. Cell-free DNA (cfDNA) testing is also available. Combining first- and second-trimester screening using integrated, sequential-stepwise, or contingent protocols offers improved detection rates compared to single-step screening.[3] Among all methods, cfDNA testing provides the highest detection rate.
Carrier screening is available to couples both during the preconception period and throughout pregnancy. This type of screening involves genetic testing on an asymptomatic individual to identify whether they carry an abnormal copy of a gene associated with a specific inherited condition. Multiple panels of genetic disorders can be tested, allowing for a broader evaluation of potential risks. Previously, carrier screening was targeted at specific ethnic groups due to the higher prevalence of certain disorders in particular populations. However, given the increasing diversity of many patient populations, ethnic-based screening is no longer recommended, as determining an individual’s specific ancestry can be challenging.
For this reason, the American College of Obstetricians and Gynecologists (ACOG) supports panethnic carrier screening. ACOG recommends that all individuals, regardless of ethnicity, be offered screening for a panel of genetic disorders.[4] In addition, ACOG advises that all individuals who are considering pregnancy or are already pregnant be offered carrier screening for conditions such as cystic fibrosis, spinal muscular atrophy, thalassemias, and hemoglobinopathies.[5] Regardless of the screening method selected, it is essential that patients receive thorough counseling on the benefits, limitations, and possible outcomes of both screening and diagnostic tests, both before and after testing.
Specimen Collection
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Specimen Collection
Prenatal genetic screening typically involves collecting a maternal blood sample via venipuncture to measure serum levels of various biomarkers, including MSAFP, pregnancy-associated plasma protein-A (PAPP-A), free beta-human chorionic gonadotropin (β-hCG), inhibin A, and unconjugated estriol. As part of first-trimester screening, a transabdominal ultrasound is usually performed between 11 and 14 weeks of gestation to assess nuchal translucency, following established measurement criteria.[6]
CfDNA testing and carrier screening are also performed using maternal blood obtained via venipuncture. CfDNA analysis is most commonly conducted using methods based on single-nucleotide polymorphisms. The fetal fraction of cfDNA originates from placental trophoblasts, which release DNA fragments into the maternal circulation. These fragments are then isolated and purified from maternal plasma for further analysis.[3]
Screening for thalassemias and hemoglobinopathies is performed using a complete blood count (CBC) obtained via maternal venipuncture. As part of this evaluation, red blood cell indices should be assessed, and hemoglobin electrophoresis should be conducted to identify potential hemoglobinopathies.
Procedures
Regardless of maternal age, all patients should be informed of the option to pursue prenatal genetic screening and diagnostic testing. This information should be communicated through an open and objective discussion that considers various factors influencing the choice of screening modality. These factors may include maternal age, the desire for specific prenatal information, prior obstetric and family history, gestational age at the time of screening, cost considerations, and the turnaround time required for results to support pregnancy-related decision-making.[3]
Screening modalities vary in terms of the gestational age at which they can be performed and the type of information they provide. First-trimester screening is limited to the window between 10 and 13 weeks, 6 days of gestation. Second-trimester triple, quadruple, and penta screening are typically conducted between 15 and 22 weeks of gestation. CfDNA testing can be performed at any time from 10 weeks of gestation onward.
First-trimester genetic screening options include the first-trimester screen, nuchal translucency measurement, and cfDNA testing. Maternal serum analytes can be measured alone or in combination with nuchal translucency. The key serum markers assessed in the first trimester are pregnancy-associated PAPP-A and free β-hCG. Nuchal translucency measurement is performed using a transabdominal ultrasound, with the fetus in a sagittal view.[7] To ensure accuracy, the fetal crown-rump length must be between 45 and 84 mm. The Fetal Medicine Foundation outlines specific criteria for obtaining accurate nuchal translucency measurements (Fetal Medicine Foundation-Nuchal Translucency Scan).
Second-trimester serum screening options include the triple, quadruple (quad), and penta screens. First- and second-trimester screenings may also be combined into integrated, sequential-stepwise, or contingent screening protocols to improve detection rates.
The triple screen measures β-hCG, MSAFP, and unconjugated estriol.[8] The quadruple screen adds inhibin A to the triple screen panel and is especially beneficial for patients who do not attend their first prenatal visit until the second trimester. The penta screen further includes hyperglycosylated hCG in addition to β-hCG, MSAFP, inhibin A, and unconjugated estriol.[3]
The integrated screen combines results from the first-trimester screening and the second-trimester quad screen into a single risk assessment, which is reported after the second-trimester screening is completed. The sequential-stepwise screen follows a similar approach but provides patients with their results from the first trimester. If the first-trimester screen is positive, diagnostic testing is offered, and further screening is typically discontinued if the test is pursued. Patients with a negative first-trimester result proceed with the second-trimester quad screen to complete the assessment.[3]
The contingent screen also provides first-trimester results but categorizes patients into low-, intermediate-, and high-risk groups based on their screening outcomes. Low-risk patients require no further testing, while high-risk patients are offered diagnostic testing. Those classified as intermediate-risk are offered additional screening with the second-trimester quad screen.[9]
Carrier screening is ideally performed during the preconception period to allow for comprehensive counseling on reproductive risks and options for future pregnancies.[10] When performed during pregnancy, screening is typically performed on the pregnant patient first. If the patient is identified as a carrier of a specific condition, the partner should be offered targeted carrier screening for that condition to assess the risk of having an affected child. In cases where time is limited, concurrent screening of both partners may be considered.[5] If both partners are found to be carriers of the same genetic condition, genetic counseling should be offered.
Indications
Prenatal screening guidelines have been issued by several major organizations, including the ACOG, the Society for Maternal-Fetal Medicine, the American College of Medical Genetics and Genomics (ACMG), and the United States Preventive Services Task Force (USPSTF). ACOG recommends that all patients, regardless of maternal age or risk factors, be offered prenatal genetic screening and diagnostic testing options. ACMG advises that patients be informed about the availability of cfDNA screening for trisomies T21, T18, and T13, as well as sex chromosome abnormalities.[11]
Prenatal genetic screening should be discussed early in pregnancy, with patients being counseled on their risk factors, the conditions being screened for, the accuracy of the screening test, and the importance of confirmatory diagnostic testing to guide pregnancy management.[2][3] Diagnostic testing is universally recommended following a positive screening result, regardless of the screening modality used.[3] If a screening test returns a low-risk or negative result, no further screening should be offered, as this may increase the likelihood of a false-positive result.
Regardless of whether screening or diagnostic testing is performed, all patients should be offered a second-trimester ultrasound to evaluate for potential fetal structural abnormalities.[3] This ultrasound is ideally conducted between 18 and 22 weeks of gestation. Some ultrasound findings are considered soft markers and should not be used solely to screen for aneuploidy, as these markers may also appear as normal variants in euploid fetuses (see Image. Soft Marker Findings on Fetal Ultrasound).[3][12]
Each soft marker should be evaluated in the clinical context of the patient, considering her baseline risk factors and prior screening results for aneuploidy. Depending on the soft marker identified, a detailed Level II anatomic survey should be performed to assess for any coexisting structural anomalies. Genetic screening and/or diagnostic options should be reviewed if already completed or offered if not yet conducted.[3]
Normal and Critical Findings
First-trimester maternal serum analyte screening, with or without nuchal translucency screening, provides results categorized as high-risk or low-risk for trisomies T21, T18, and T13. In general, PAPP-A levels are decreased in the presence of trisomies T21, T18, and T13. β-hCG is typically decreased in T18 and T13 but increased in T21. A nuchal translucency measurement greater than 3 mm is considered abnormal and, when used in combination with serum analyte screening, can help increase the risk assessment for aneuploidy.
Second-trimester maternal serum analyte screening results are categorized as low-risk or high-risk based on characteristic analyte patterns associated with specific trisomies:
- T21 is associated with low MSAFP, low estriol, high β-hCG, high inhibin A, and low PAPP-A.
- T18 is associated with low MSAFP, low estriol, low β-hCG, normal inhibin A, and low PAPP-A.
- T13 is associated with normal MSAFP, normal estriol, normal β-hCG, normal inhibin A, and low PAPP-A.
cfDNA screening provides individual results reported as either high-risk or low-risk for each specific genetic condition tested, including trisomies T21, T18, and T13, as well as sex chromosome abnormalities and particular microdeletions, including 22q11.2. In some cases, cfDNA results may return as a "no call," indicating that a determination of risk could not be made for any of the conditions tested. The ACMG recommends that all "no call" cfDNA reports include the fetal fraction.[3][13]
Interfering Factors
CfDNA screening is dependent on a sufficient fetal fraction—the proportion of fetal DNA in the maternal blood sample—for accurate analysis. The minimum fetal fraction required for reliable cfDNA screening is generally considered to be between 2% and 4%, depending on the specific screening test and laboratory used. Maternal weight can affect the fetal fraction.
Approximately 10% of women with a body weight of over 250 pounds may have a fetal fraction of less than 4%, which can lead to test failure. In addition to maternal body mass index (BMI), test failure can also occur due to factors such as the type of laboratory analysis used by a specific genetic screening company, early gestational age at the time of collection, advanced maternal age, pregnancies conceived via in vitro fertilization, maternal use of low molecular weight heparin, and certain racial backgrounds, including Black and South Asian women.[3]
Test results that cannot be calculated (no call results) due to these interfering factors should not be considered equivalent to low-risk results.[14] Patients should be counseled about the ongoing possibility of a chromosomal abnormality and offered genetic diagnostic testing as a means to confirm or rule out this possibility. Inaccurate test results may also occur in cases of multiple gestations, particularly when one twin has been lost early (vanishing twin) or when one twin has a genetic abnormality while the co-twin is genetically normal. Specific cfDNA tests are available for use in the context of a vanishing twin.[15]
Complications
Standard phlebotomy for obtaining maternal blood samples carries risks such as bruising, bleeding, phlebitis, and localized pain. Ultrasonography, in use for over 30 years, is considered a very low-risk procedure for both the mother and fetus. The "as low as reasonably achievable" (ALARA) principle has been established to ensure the safety of radiological imaging modalities.[16]
CfDNA is the most sensitive and specific of the genetic screening modalities, but still carries a small risk of false-negative or false-positive results. Serum analyte screening modalities are generally considered to carry a 5% risk of false-positive results.[3] The potential for patient anxiety and distress resulting from a false-positive result should be thoughtfully considered and addressed during pretest counseling. In cfDNA screening specifically, incidental findings may be reported and can be associated with maternal aneuploidy, mosaicism, or malignancy.[3] This information should also be included in the pretest counseling as a possible complication of prenatal genetic screening.
The information presented so far applies to singleton gestations. Serum aneuploidy screening, regardless of the method used, is less accurate in twin pregnancies compared to singleton gestations. Reliable data regarding genetic screening in higher-order multiples, such as triplets and quadruplets, is lacking. First-trimester screening, including serum analytes and nuchal translucency, as well as second-trimester quad screening and sequential-stepwise or integrated screening, are available options for twin pregnancies.[3] CfDNA can also be performed in twin gestations. However, interpreting the results in twin pregnancies requires careful consideration, as it is not possible to determine which twin may be affected based on blood work alone.[17]
Counseling patients about carrier screening is essential, as it cannot identify all individuals who may be at risk for the condition being screened for. Many gene variations associated with a given condition may not be included in the screening. Patients should be informed that a residual risk remains with any genetic testing result.[4]
Patient Safety and Education
Patient safety and education play a crucial role in prenatal screening. Pretest and posttest counseling are essential to ensure that patients fully understand the purpose of screening tests and the distinction between screening and diagnostic testing. A positive screening result should always be followed by diagnostic testing before any irreversible decisions are made regarding the pregnancy.
Additionally, patients must be informed that a negative or low-risk result from a screening test does not entirely rule out the possibility of an abnormality, as there may still be residual risk. Counseling should also include a discussion of test sensitivity, specificity, and positive predictive values to help patients interpret the results accurately. For patients with concerns about the implications of genetic testing and the privacy of their results, it is important to provide information about the Genetic Information Nondiscrimination Act of 2008.[18]
Clinical Significance
Identifying fetal anomalies and genetic conditions during the prenatal period offers several significant advantages. Early detection allows parents and healthcare providers to make informed decisions and prepare for potential outcomes. This may include transferring care and planning delivery at a facility equipped with a neonatal intensive care unit and pediatric subspecialists to support a newborn with genetic or structural abnormalities. In addition, early detection also provides families with time for emotional preparation and planning for the potential long-term needs of the child. Additionally, early diagnosis may offer the option of pregnancy termination, if desired.[19]
Media
(Click Image to Enlarge)
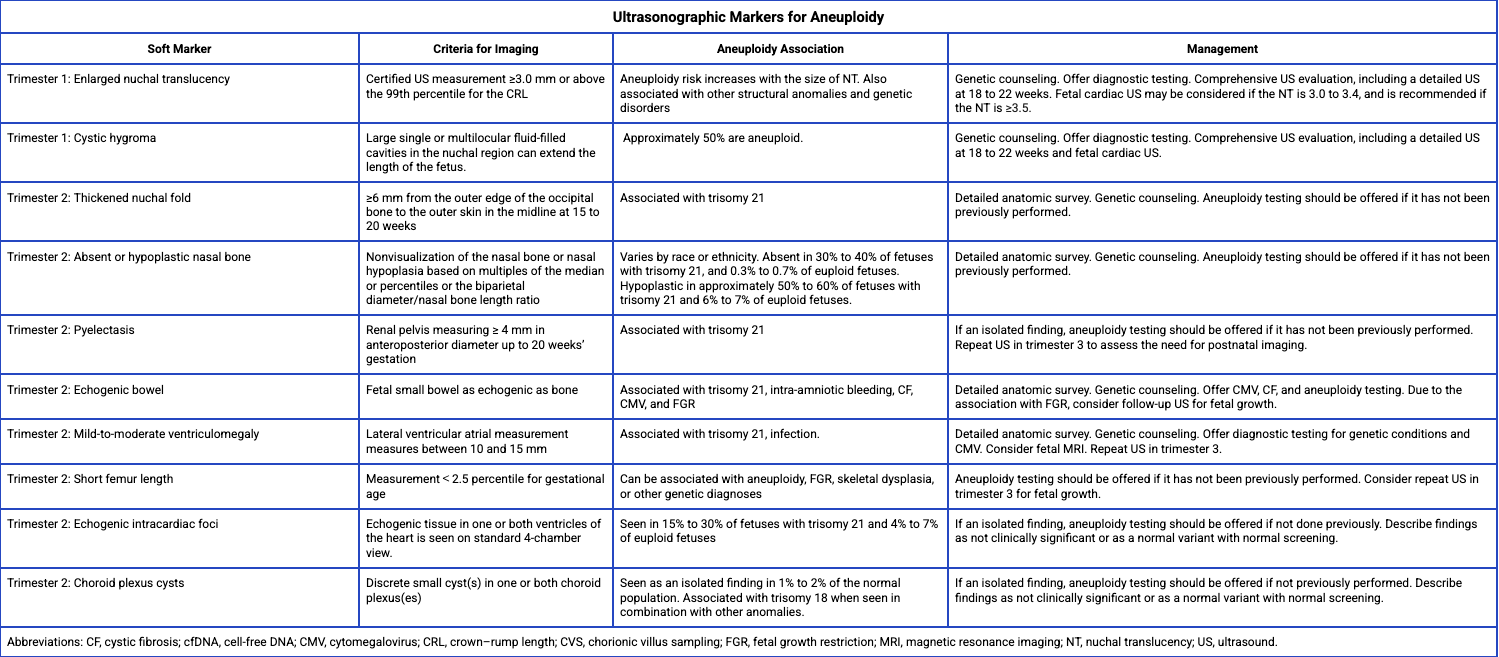
Soft Marker Findings on Fetal Ultrasound. The image shows associations with fetal aneuploidy and recommended management strategies.
Adapted from the American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics; Committee on Genetics; Society for Maternal-Fetal Medicine. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet Gynecol. 2020;136(4):e48-e69.
References
Cuckle H, Maymon R. Development of prenatal screening--A historical overview. Seminars in perinatology. 2016 Feb:40(1):12-22. doi: 10.1053/j.semperi.2015.11.003. Epub 2016 Jan 4 [PubMed PMID: 26764253]
Level 3 (low-level) evidence. Practice Bulletin No. 162: Prenatal Diagnostic Testing for Genetic Disorders. Obstetrics and gynecology. 2016 May:127(5):e108-e122. doi: 10.1097/AOG.0000000000001405. Epub [PubMed PMID: 26938573]
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics, Committee on Genetics, Society for Maternal-Fetal Medicine. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstetrics and gynecology. 2020 Oct:136(4):e48-e69. doi: 10.1097/AOG.0000000000004084. Epub [PubMed PMID: 32804883]
. Committee Opinion No. 690: Carrier Screening in the Age of Genomic Medicine. Obstetrics and gynecology. 2017 Mar:129(3):e35-e40. doi: 10.1097/AOG.0000000000001951. Epub [PubMed PMID: 28225425]
Level 3 (low-level) evidence. Committee Opinion No. 691: Carrier Screening for Genetic Conditions. Obstetrics and gynecology. 2017 Mar:129(3):e41-e55. doi: 10.1097/AOG.0000000000001952. Epub [PubMed PMID: 28225426]
Level 3 (low-level) evidenceAbuhamad A. Technical aspects of nuchal translucency measurement. Seminars in perinatology. 2005 Dec:29(6):376-9 [PubMed PMID: 16533650]
Hixson L, Goel S, Schuber P, Faltas V, Lee J, Narayakkadan A, Leung H, Osborne J. An Overview on Prenatal Screening for Chromosomal Aberrations. Journal of laboratory automation. 2015 Oct:20(5):562-73. doi: 10.1177/2211068214564595. Epub 2015 Jan 13 [PubMed PMID: 25587000]
Level 3 (low-level) evidenceMalone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, Berkowitz RL, Gross SJ, Dugoff L, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, Dukes K, Bianchi DW, Rudnicka AR, Hackshaw AK, Lambert-Messerlian G, Wald NJ, D'Alton ME, First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. First-trimester or second-trimester screening, or both, for Down's syndrome. The New England journal of medicine. 2005 Nov 10:353(19):2001-11 [PubMed PMID: 16282175]
Carlson LM, Vora NL. Prenatal Diagnosis: Screening and Diagnostic Tools. Obstetrics and gynecology clinics of North America. 2017 Jun:44(2):245-256. doi: 10.1016/j.ogc.2017.02.004. Epub [PubMed PMID: 28499534]
Fu XL, Hou W, Zhang ML, Xie XX, Meng Y, Zhou HH, Zhao QD, Hu JL, Mo GP, Lu YP. [Carrier screening and prenatal diagnosis analysis of high-risk cases in 3 044 preconception and early pregnancy couples]. Zhonghua fu chan ke za zhi. 2025 Mar 25:60(3):161-170. doi: 10.3760/cma.j.cn112141-20240928-00531. Epub [PubMed PMID: 40159040]
Level 3 (low-level) evidenceGregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, Klugman S, Watson MS. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genetics in medicine : official journal of the American College of Medical Genetics. 2016 Oct:18(10):1056-65. doi: 10.1038/gim.2016.97. Epub 2016 Jul 28 [PubMed PMID: 27467454]
Temming LA, Macones GA. What is prenatal screening and why to do it? Seminars in perinatology. 2016 Feb:40(1):3-11. doi: 10.1053/j.semperi.2015.11.002. Epub 2015 Dec 18 [PubMed PMID: 26708051]
Post AL, Mottola AT, Kuller JA. What's New in Prenatal Genetics? A Review of Current Recommendations and Guidelines. Obstetrical & gynecological survey. 2017 Oct:72(10):610-617. doi: 10.1097/OGX.0000000000000491. Epub [PubMed PMID: 29059453]
Lannoo L, Van Den Bogaert K, Belmans A, Brison N, Dehaspe L, De Langhe E, Vancoillie L, Parijs I, Vermeesch JR, Devriendt K, Van Calsteren K. Persistent Uninterpretable or Failed Prenatal Cell-Free DNA Screening Indicates a High-Risk Pregnancy and is Associated With Biological Factors Interfering With cfDNA-Analysis: A Prospective Cohort Study. Prenatal diagnosis. 2025 Mar 20:():. doi: 10.1002/pd.6778. Epub 2025 Mar 20 [PubMed PMID: 40114366]
Curnow KJ, Wilkins-Haug L, Ryan A, Kırkızlar E, Stosic M, Hall MP, Sigurjonsson S, Demko Z, Rabinowitz M, Gross SJ. Detection of triploid, molar, and vanishing twin pregnancies by a single-nucleotide polymorphism-based noninvasive prenatal test. American journal of obstetrics and gynecology. 2015 Jan:212(1):79.e1-9. doi: 10.1016/j.ajog.2014.10.012. Epub 2014 Oct 15 [PubMed PMID: 25447960]
Varthaliti A, Fasoulakis Z, Lygizos V, Zolota V, Chatziioannou MI, Daskalaki MA, Daskalakis G, Antsaklis P. Safety of Obstetric Ultrasound: Mechanical and Thermal Indexes-A Systematic Review. Journal of clinical medicine. 2024 Nov 1:13(21):. doi: 10.3390/jcm13216588. Epub 2024 Nov 1 [PubMed PMID: 39518728]
Level 1 (high-level) evidenceJudah H, Gil MM, Syngelaki A, Galeva S, Jani J, Akolekar R, Nicolaides KH. Cell-free DNA testing of maternal blood in screening for trisomies in twin pregnancy: updated cohort study at 10-14 weeks and meta-analysis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2021 Aug:58(2):178-189. doi: 10.1002/uog.23648. Epub 2021 Jul 10 [PubMed PMID: 33838069]
Level 1 (high-level) evidence. Committee Opinion No. 693: Counseling About Genetic Testing and Communication of Genetic Test Results. Obstetrics and gynecology. 2017 Apr:129(4):e96-e101. doi: 10.1097/AOG.0000000000002020. Epub [PubMed PMID: 28333821]
Level 3 (low-level) evidenceDukhovny S, Norton ME. What are the goals of prenatal genetic testing? Seminars in perinatology. 2018 Aug:42(5):270-274. doi: 10.1053/j.semperi.2018.07.002. Epub 2018 Jul 26 [PubMed PMID: 30195989]