Introduction
Chronic traumatic encephalopathy (CTE), formerly known as repetitive head injury syndrome, is a progressive neurodegenerative condition commonly observed in individuals involved in contact sports or military service associated with an increased risk of repeated head injuries. This condition is characterized by regional atrophy, ventriculomegaly, and specific brain abnormalities.[1] Historically, CTE has been linked to repeated head trauma, with early descriptions highlighting its association with "punch drunk" syndrome and ''dementia pugilistica" in boxers. The condition has evolved into a significant topic within the sports medicine community, with ongoing investigations into its pathophysiology, diagnostic criteria, and potential therapeutic approaches.[1][2]
Historical Background
Harrison Martland was the first to mention "perivascular microhemorrhages" that progressively evolved into "replacement gliosis," resulting in punch drunk syndrome. Abram Bowman and Karl Blau then coined the term CTE to better characterize the condition. N. Corsellis subsequently identified neurofibrillary tangles (NFTs), along with ventricular dilatation, cavum septum pellucidum, thinning of the corpus callosum, and cerebellar tonsillar scarring in affected individuals. Additional neuropathological changes in CTE were later documented by Jennian Geddes, who noted perivascular neurofibrillary encasement within cortical sulci, and Bennet Omalu, who described amyloid plaques, tau-positive neurofibrillary tangles, and neuropil threads associated with the condition.
Ann McKee proposed neuropathological diagnostic and grading criteria for CTE, characterized by patchy deposits of phosphorylated tau (p-tau)–positive NFTs and astrocytic tangles located perivascularly within the neocortex, at the depths of cerebral sulci, and in the superficial layers of the cortex, primarily in the temporal lobe. The National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have established criteria for diagnosing CTE, focusing on perivascular foci of p-tau NFTs and astrocytic tangles in the cortex, with a preference for their presence at sulcal depths and in the superficial layers of the cerebral cortex.
In 2021, the panel refined the definition of the pathognomonic lesion to emphasize that perivascular p-tau aggregates must involve neurons and extend deeper than the subpial layer. Additionally, the panel introduced the classifications "Low CTE" and "High CTE" to indicate the presence of NFTs in specific brain regions, such as the thalamus, mammillary bodies, hippocampus, amygdala, and entorhinal cortex, to assist neuropathologists in their assessments.[3]
While CTE can only be definitively diagnosed through postmortem examination, recent consensus guidelines have established criteria for identifying its clinical manifestations, referred to as traumatic encephalopathy syndrome (TES).[4] Montenegro et al proposed the clinical diagnostic criteria for CTE comprising cognitive, behavioral, and mood symptomatology as 3 core elements alongside 9 other supportive features, including impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs, documented decline, and delayed onset.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Repeated head injury is crucial in the genesis of CTE, with a strong causal relationship established between repeated trauma and the condition.[3] Repeated head injury exposure is the only known unifying factor and is present in 97% of patients among the more than 600 CTE cases reported in the literature.[3] Computational models and animal studies involving Musk oxen and bighorn sheep—both with gyrencephalic brains (with sulci) and engaged in combative headbutting—have shown positive correlations between repeated head injury and CTE.[3]
An association exists between cognitive reserve, demographic factors (eg, early age of first exposure), and exposure metrics (eg, cumulative head impact index) with the severity of future neurological sequelae.[1][6] A consistent dose-response relationship has been validated between cumulative years of playing contact sports, such as American football and ice hockey, and both the onset and severity of CTE.[3] Additionally, the APOE epsilon-4 allele has been shown to predict an increased risk of developing cognitive decline following repeated head impacts.[1]
Epidemiology
Approximately 4 million sports-related concussions are reported annually in the United States alone.[1] The incidence of sport-related neurological conditions among boxers is reported to be 17%. In a study conducted by the Mayo Clinic Brain Bank, CTE pathology was found in 32% of athletes who participated in contact sports. Similarly, in the largest case series of 177 former professional football players, CTE was diagnosed in up to 87% of the patients.[1] A recent study has also revealed significant neuropathological evidence of CTE among football players who donated their brains for research.[7]
Pathophysiology
Tau protein regulates the assembly of tubulin into microtubules, thereby maintaining the structural integrity of axons. Tau phosphorylation mediates tau's binding to microtubules. Tau proteins dissociate from microtubules in axons due to calcium influx and glutamate hyperexcitotoxicity. Calcium influx activates caspases, while glutamate hyperexcitotoxicity causes cytoskeletal failure. These disruptions lead to kinase-mediated hyperphosphorylation, misfolding, and aggregation of tau, which is then proteolytically cleaved by calpains and caspases.[8][9] These processes, including tau phosphorylation, misfolding, shortening, and aggregation, contribute to NFT deposition.[9]
TAR DNA-binding protein 43 (TDP-43) inclusions and amyloid-β deposition are also observed in CTE. Perivascular polarization of astroglial aquaporin-4 impairs glymphatic clearance.[9] The combination of excessive protein deposition and reduced clearance aggravates the neurodegenerative process. Oxidative stress, neuroinflammation, and glutaminergic toxicity contribute significantly to CTE pathogenesis. Chronic inflammation disrupts the ubiquitin-proteasome pathway, while microglial priming leads to immune-excitotoxic responses and persistent neurodegeneration.[9]
Immuno-excitotoxicity is critical in the pathogenesis of CTE. Repeatedly primed microglia, after a repetitive head injury, fail to transition from a neuro-destructive mode to a reparative mode. This failure exacerbates the inflammatory insult to the brain by providing a favorable milieu amidst glutamate toxicity.[10] Excitotoxicity also generates reactive oxygen and nitrogen species.[9] Repeated head injury causes axonal degeneration and microtubular disintegration, leading to tau oligomerization. This oligomerization progresses to the formation of NFTs, affecting neuronal crosstalk and networking. This disruption eventually results in tau propagation, which triggers inflammatory cascades and ultimately impairs blood-brain barrier permeability.[11]
Histopathology
Gross examination reveals features of regional atrophy, most commonly in the frontal lobe, along with ventriculomegaly, wasting of the corpus callosum, and characteristic fenestration of the septum pellucidum. The regions of atrophy parallel high concentrations of glutamate receptors, thereby connoting excitotoxicity's role in the entity's pathogenesis.[1]
Microscopically, the predominant findings include the deposition of NFTs and neuropil threads. In CTE, NFTs are deposited perivascularly at the sulcal depths and are exclusively neuronal.[3][12] 4R isomers are predominant in stages I and II, while 3R isomers are more prevalent in stages III and IV.[3] Perivascular tau deposition within the sulcal depths is a critical criterion for diagnosing CTE, according to the first consensus from the NINDS and NIBIB.[1] The presence of neuronal p-tau is significantly associated with age, years of repeated head injury exposure, and the severity of CTE.[3]
The following 4 histomorphologic phenotypes have been associated with CTE:
- Type 1: NFTs and neuropil threads are present only in the cerebral cortex and brainstem.
- Type 2: Type 1 features along with amyloid-β deposition.
- Type 3: NFTs and neuropil threads are found exclusively in the brainstem.
- Type 4: NFTs and neuropil threads in the cortex, subcortex, brainstem, and basal ganglia, with the cerebellum being spared.[13]
The Understanding Neurologic Injury in Traumatic Encephalopathy (UNITE) study, funded by the NINDS, also described patchy and perivascular deposits of NFTs in astrocytes and at the depths of the sulci.[14] As established by the NINDS panel consensus, the pattern of p-tau in CTE is distinct from other neurodegenerative conditions.[14] Hallmarks include the superficial distribution of NFTs in layers II and III, patchy distribution of NFTs, and a perivascular pattern within the depths of the cortical sulci.[3] Brainstem p-tau pathology is considered a supportive feature of CTE.[3] P-tau spares the calcarine cortex even in the advanced stage.[9]
History and Physical
The medical history typically includes some form of repetitive head trauma, often involving individuals who participate in contact sports or serve in the military. A thorough neurologic examination is crucial, with a particular emphasis on mental status assessment.[1] Younger individuals are more likely to present with mood and behavior symptoms, while older individuals commonly exhibit cognitive impairment and executive dysfunction.[3] Cognitive symptoms increase the odds of CTE by 3.6-fold.[14] During longitudinal follow-up, patients with a history of recurrent concussions tend to have a higher burden of cognitive, sleep, and neuropsychiatric symptoms but not migraine symptoms.[2]
As described by Browne, the disease process begins with affective disturbances, followed by a stage of social instability and behavioral changes, with subtle features of early Parkinsonism. This eventually progresses to a third stage characterized by cognitive dysfunction, dementia, and full-blown Parkinsonism.[15] These changes have been attributed to the disease's impact on the Papez circuit.[16]
The research diagnostic criteria describe 4 subtypes of CTE, including:
- Behavioral or mood variant
- Cognitive variant
- Mixed variant
- Dementia form [1]
Evaluation
Montenegro et al proposed the following clinical diagnostic criteria for CTE:
- A history of multiple head impacts
- Exclusion of other clinical mimics
- Symptomatology present for at least 12 months
- A minimum of 1 core and 2 supportive elements present[17]
The core elements comprise cognitive symptoms (eg, episodic memory, executive function, and attention), behavioral (eg, verbal or physical aggression), and mood symptoms (eg, feeling depressed or hopeless).[14] As CTE can only be definitively diagnosed through postmortem neuropathologic findings, the NINDS consensus developed guidelines to aid in the clinical identification of TES.[4]
The 4 primary diagnostic criteria proposed by the NINDS consensus for TES include:
- Substantial exposure to repeated head injury
- Core clinical features such as cognitive impairment (eg, episodic memory and executive functioning) and neurobehavioral dysregulation (eg, explosiveness, impulsivity, rage, violent outbursts, and emotional lability) with a progressive course
- Clinical features not fully accounted for by other disorders [3]
However, these criteria have a high risk of false positives, with nearly 54% of cognitively normal individuals receiving a consensus diagnosis. Additionally, there is a lack of spatial correlation between TES and head injury exposure.[18] The degree of depression is the only significant predictor of a positive TES diagnosis.[18]
Although CTE is confirmed through postmortem autopsy, the diagnosis is facilitated by immunohistochemistry for p-tau.[6][11] Recent advances in imaging armamentarium and fluid biomarkers can also provide valuable input to support the diagnosis.[11][19][20] Growing evidence supports the role of the regional tau standardized uptake value ratio in flortaucipir positron-emission tomography (PET) and florbetapir PET for facilitating diagnosis,[14] although the specificity remains poorly documented.[21] Specific prototypical patterns or regional volume differences have not been identified in PET imaging.[22]
A significant correlation has been observed only in the superior frontal region and among individuals aged 60 or older.[23] Furthermore, off-target binding in the hippocampus and thalamus complicates the interpretation of imaging results.[21] Other important radiological features include concurrent cavum septum pellucidum and reduced fractional anisotropy in the corpus callosum and medial temporal white matter.[14][22] Magnetic resonance imaging (MRI) and PET imaging are essential for ruling out other clinical mimics.[22]
Potential fluid biomarkers for CTE include glial fibrillary acidic protein (GFAP), neurofibrillary light chain (NfL), total tau, neuron-specific enolase (NSE), ubiquitin C-terminal hydrolase-1 (UCHL-1), S100B, myelin basic protein (MBP), microtubule-associated protein-2 (MAP-2), brain-derived neurotrophic factor (BDNF), microRNA, and microvesicles and exosomes.[14][24][25] However, plasma biomarkers do not correspond well with cerebrospinal fluid measures in cases of late repetitive head injury, thereby minimizing their efficacy.[26] Therefore, consensus on the role of fluid biomarkers in managing CTE has yet to be established. However, a recent pivotal finding of a characteristic hydrophobic cavity within the β-helix of tau filaments from CTE has opened new avenues for identifying early diagnostic targets (see Image. Chronic Traumatic Encephalopathy).[27]
Treatment / Management
Currently, a definitive treatment for CTE does not exist; therefore, multispectral supportive measures are the mainstay of management.[1] Amantadine and guanfacine may offer benefits for cognitive and working memory deficits. Cognitive improvement can be supported through cognitive rehabilitation therapy, a Mediterranean diet, and aerobic exercise.[14] Occupational rehabilitation should also be encouraged. Depression requires careful management due to the potential risk of suicidality.
Antioxidants such as ascorbic acid, N-acetylcysteine, alpha-tocopherol (ie, vitamin E), carotenoids, and omega-3 fatty acids have been used to counteract reactive oxygen species and reactive nitrogen species. Salsalate, which inhibits the acetylation process before the phosphorylation of the paired helical filament-6 motif and thereby suppresses microglial activation, is under research.[11] Ongoing research also focuses on tau acetylation, tau phosphorylation, and immunotherapy, such as using adeno-associated virus vectors to deliver anti-p-tau antibodies.
The best modality for minimizing the incidence of CTE is through strict adherence to preventive measures and safe practices.[14] Establishing mandatory provisions for a safe playing environment and strictly upholding "return-to-play" policies are paramount.[12]
Differential Diagnosis
In addition to a single traumatic brain injury, conditions with clinical features that mimic CTE include:[28][29]
- Alzheimer disease.
- Frontotemporal lobar degeneration.
- Lewy body disease.
- Cerebral amyloid angiopathy.
- Parkinsonism.
- Age-related tau astrogliopathy, characterized by subpial "thorn-shaped astrocytes" with purely astrocytic perivascular p-tau pathology, is observed. Moreover, 47% of these lesions are restricted to the midbrain, whereas current criteria for CTE require at least 1 pathognomonic cortical lesion.
- Limbic-predominant age-related TDP-43 encephalopathy with neuropathologic changes associated with hippocampal sclerosis.
- Bulbar amyotrophic lateral sclerosis.
- Primary age-related tauopathy (PART).[3][30]
The characteristic finding that helps differentiate CTE from other tauopathies, such as Alzheimer and Lewy body dementia, is the perivascular deposition of tau-immunoreactive astrocytes within the sulcal depths of superficial cortical layers.[11] Additionally, the dimensions of NFTs in CTE are larger and are associated with co-localized aggregates of TDP-43.[11] Concurrent neurodegenerative diseases are observed in almost 40% of CTE cases.[3] However, CTE remains a unique tauopathy—both ultrastructurally and microscopically.[3]
The tau filament in CTE features a unique β-helix region with a hydrophobic cavity.[14][27] CTE exhibits higher levels of p-tau in the CA2 and CA3 regions of the hippocampus compared to CA1 and subiculum in Alzheimer disease. Similarly, CA3 and CA4 regions have significantly higher p-tau burden in CTE than PART.[3]
Pertinent Studies and Ongoing Trials
NEurodegeneration: Traumatic Brain Injury as Origin of the Neuropathology (NEwTON)
NEwTON is a prospective study recruiting patients at risk for developing CTE.[31] Similarly, the "Diagnostics, Imaging, and Genetics Network for the Objective Study and Evaluation of Chronic Traumatic Encephalopathy (DIAGNOSE CTE)'' research project aims to provide newer insights into CTE's pathogenesis and natural history.[32]
Staging
The following 4 stages of CTE are described according to the distribution of NFTs and associated clinical signs and symptoms:
- Stage I: Perivascular deposits of NFTs in the sulcal depths characterized by headache and loss of concentration.
- Stage II: Stage I features plus NFTs within the nucleus basalis of Meynert and locus coeruleus, with mood swings and short-term memory loss.
- Stage III: Stage II features plus atrophy, wasting of the corpus callosum, septal abnormalities, ventriculomegaly, depigmentation of the substantia nigra, and widespread deposition of p-tau, characterized by cognitive impairment, executive dysfunction, and visuospatial abnormalities.
- Stage IV: Stage III features plus further atrophy, gliosis, and hippocampal sclerosis, characterized by profound memory loss and florid parkinsonian features.[1]
McKee described the following 4 pathological stages of CTE:
- Stage I: Perivascular p-tau deposits in the sulci, primarily within the superior dorsolateral and inferior frontal cortices.
- Stage II: Mild ventricular enlargement and changes in the septum pellucidum.
- Stage III: Progressive ventricular enlargement, mild frontal and temporal atrophy, and NFT deposits within the olfactory bulb, entorhinal cortex, hippocampus, amygdala, and mammillary bodies.
- Stage IV: Diffuse cortical atrophy and complete depigmentation of the locus coeruleus and substantia nigra.[6]
Prognosis
The incidence of mortality among former players has been observed to be 3 times higher than their healthy counterparts. Furthermore, studies have demonstrated a link between CTE and the development of early-onset Parkinsonian dementia.[33]
Complications
CTE is progressive in approximately 68% of patients. In a small subgroup with predominantly behavioral or mood symptoms, the condition may remain stable for years before progressing to other stages after a latency of 11 to 14 years.[1] The neurodegenerative process sequentially progresses through stages of social instability and behavioral changes before advancing to dementia.[1] Additionally, there is an increased risk of suicide among these subsets of patients.
Deterrence and Patient Education
Preventive measures for high-risk cohorts are the cornerstone of CTE management. Promoting a safe playing environment, strict adherence to concussion reporting, early diagnosis, and mandatory implementation of the "return-to-play" protocol are crucial in minimizing the incidence of CTE.[5]
Pearls and Other Issues
Despite recent attention and resources devoted to CTE, significant progress in understanding the disease remains limited. Many perspectives continue to rely on assumptions rather than facts, as Corsellis argued 4 decades ago.[15] However, tau imaging and relevant cerebrospinal fluid biomarkers have shown promise as potential surrogate markers for the supportive diagnosis of CTE.[1]
Enhancing Healthcare Team Outcomes
An interprofessional team approach is essential for enhancing patient-centered care and outcomes for individuals with CTE. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must collaborate to develop a comprehensive strategy emphasizing teamwork and communication. Given the inherent heterogeneity of CTE, healthcare team members should leverage their expertise to create individualized care plans that address each patient's unique needs. This approach includes implementing effective concussion reporting systems and adhering to "return-to-play" protocols to ensure safety for high-risk populations.
Additionally, ongoing education and training in cognitive rehabilitation techniques can empower healthcare clinicians to deliver effective cognitive interventions. As research advances in targeting tau protein oligomerization and propagation, the healthcare team must stay informed about emerging therapies and biomarkers, facilitating timely interventions. Regular interprofessional meetings can foster open communication, allowing healthcare team members to discuss patient progress, share insights from longitudinal studies, and coordinate care effectively. By prioritizing patient safety and team performance, healthcare professionals can significantly improve the management of CTE, ultimately enhancing the quality of care and outcomes for affected individuals.
Media
(Click Image to Enlarge)
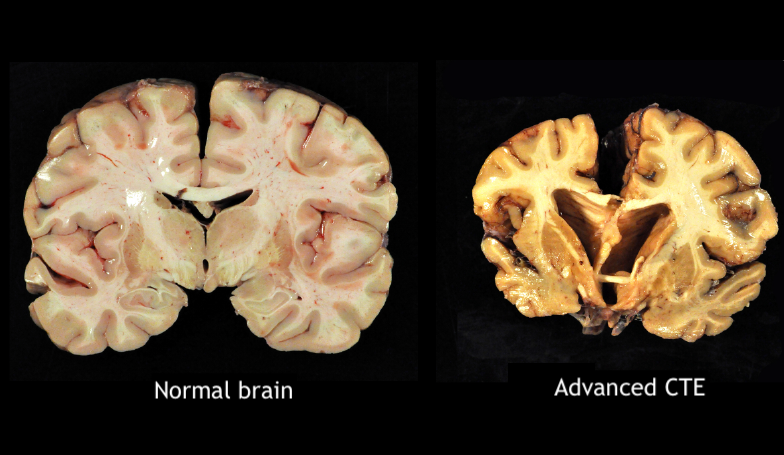
Chronic Traumatic Encephalopathy. The brain dissection images show a normal brain on the left and a brain with stage IV chronic traumatic encephalopathy (CTE) on the right.
Boston University Chronic Traumatic Encephalopathy Center and Brain Bank., Public Domain, via Wikimedia Commons
References
Turk KW, Budson AE. Chronic Traumatic Encephalopathy. Continuum (Minneapolis, Minn.). 2019 Feb:25(1):187-207. doi: 10.1212/CON.0000000000000686. Epub [PubMed PMID: 30707193]
Miyata M, Takahata K. [Challenges of Diagnostic Imaging of Chronic Traumatic Encephalopathy]. Brain and nerve = Shinkei kenkyu no shinpo. 2023 Jun:75(6):769-778. doi: 10.11477/mf.1416202413. Epub [PubMed PMID: 37287361]
McKee AC, Stein TD, Huber BR, Crary JF, Bieniek K, Dickson D, Alvarez VE, Cherry JD, Farrell K, Butler M, Uretsky M, Abdolmohammadi B, Alosco ML, Tripodis Y, Mez J, Daneshvar DH. Chronic traumatic encephalopathy (CTE): criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta neuropathologica. 2023 Apr:145(4):371-394. doi: 10.1007/s00401-023-02540-w. Epub 2023 Feb 10 [PubMed PMID: 36759368]
Katz DI, Bernick C, Dodick DW, Mez J, Mariani ML, Adler CH, Alosco ML, Balcer LJ, Banks SJ, Barr WB, Brody DL, Cantu RC, Dams-O'Connor K, Geda YE, Jordan BD, McAllister TW, Peskind ER, Petersen RC, Wethe JV, Zafonte RD, Foley ÉM, Babcock DJ, Koroshetz WJ, Tripodis Y, McKee AC, Shenton ME, Cummings JL, Reiman EM, Stern RA. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology. 2021 May 4:96(18):848-863. doi: 10.1212/WNL.0000000000011850. Epub 2021 Mar 15 [PubMed PMID: 33722990]
Level 3 (low-level) evidenceEaton RG, Lonser RR. History of biological, mechanistic, and clinical understanding of concussion. Neurosurgical focus. 2024 Jul:57(1):E2. doi: 10.3171/2024.5.FOCUS24149. Epub [PubMed PMID: 38950436]
Level 3 (low-level) evidenceArciniega H, Baucom ZH, Tuz-Zahra F, Tripodis Y, John O, Carrington H, Kim N, Knyazhanskaya EE, Jung LB, Breedlove K, Wiegand TLT, Daneshvar DH, Rushmore RJ, Billah T, Pasternak O, Coleman MJ, Adler CH, Bernick C, Balcer LJ, Alosco ML, Koerte IK, Lin AP, Cummings JL, Reiman EM, Stern RA, Shenton ME, Bouix S. Brain morphometry in former American football players: findings from the DIAGNOSE CTE research project. Brain : a journal of neurology. 2024 Oct 3:147(10):3596-3610. doi: 10.1093/brain/awae098. Epub [PubMed PMID: 38533783]
Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, Alosco ML, Solomon TM, Nowinski CJ, McHale L, Cormier KA, Kubilus CA, Martin BM, Murphy L, Baugh CM, Montenigro PH, Chaisson CE, Tripodis Y, Kowall NW, Weuve J, McClean MD, Cantu RC, Goldstein LE, Katz DI, Stern RA, Stein TD, McKee AC. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA. 2017 Jul 25:318(4):360-370. doi: 10.1001/jama.2017.8334. Epub [PubMed PMID: 28742910]
Castellani RJ, Perry G. Tau Biology, Tauopathy, Traumatic Brain Injury, and Diagnostic Challenges. Journal of Alzheimer's disease : JAD. 2019:67(2):447-467. doi: 10.3233/JAD-180721. Epub [PubMed PMID: 30584140]
Ruchika F, Shah S, Neupane D, Vijay R, Mehkri Y, Lucke-Wold B. Understanding the Molecular Progression of Chronic Traumatic Encephalopathy in Traumatic Brain Injury, Aging and Neurodegenerative Disease. International journal of molecular sciences. 2023 Jan 17:24(3):. doi: 10.3390/ijms24031847. Epub 2023 Jan 17 [PubMed PMID: 36768171]
Level 3 (low-level) evidenceBlaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surgical neurology international. 2011:2():107. doi: 10.4103/2152-7806.83391. Epub 2011 Jul 30 [PubMed PMID: 21886880]
Tharmaratnam T, Iskandar MA, Tabobondung TC, Tobbia I, Gopee-Ramanan P, Tabobondung TA. Chronic Traumatic Encephalopathy in Professional American Football Players: Where Are We Now? Frontiers in neurology. 2018:9():445. doi: 10.3389/fneur.2018.00445. Epub 2018 Jun 19 [PubMed PMID: 29971037]
Saulle M, Greenwald BD. Chronic traumatic encephalopathy: a review. Rehabilitation research and practice. 2012:2012():816069. doi: 10.1155/2012/816069. Epub 2012 Apr 10 [PubMed PMID: 22567320]
Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011 Jul:69(1):173-83; discussion 183. doi: 10.1227/NEU.0b013e318212bc7b. Epub [PubMed PMID: 21358359]
Level 2 (mid-level) evidenceFesharaki-Zadeh A. Navigating the Complexities of Traumatic Encephalopathy Syndrome (TES): Current State and Future Challenges. Biomedicines. 2023 Nov 27:11(12):. doi: 10.3390/biomedicines11123158. Epub 2023 Nov 27 [PubMed PMID: 38137378]
Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychological medicine. 1973 Aug:3(3):270-303 [PubMed PMID: 4729191]
Eggers AE. Redrawing Papez' circuit: a theory about how acute stress becomes chronic and causes disease. Medical hypotheses. 2007:69(4):852-7 [PubMed PMID: 17376605]
Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, Katz DI, Cantu RC, Stern RA. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer's research & therapy. 2014:6(5):68. doi: 10.1186/s13195-014-0068-z. Epub 2014 Sep 24 [PubMed PMID: 25580160]
Schaffert J, Didehbani N, LoBue C, Hart J, Rossetti H, Lacritz L, Cullum CM. Frequency and Predictors of Traumatic Encephalopathy Syndrome in a Prospective Cohort of Retired Professional Athletes. Frontiers in neurology. 2021:12():617526. doi: 10.3389/fneur.2021.617526. Epub 2021 Feb 23 [PubMed PMID: 33708171]
Cherry JD, Stein TD, Tripodis Y, Alvarez VE, Huber BR, Au R, Kiernan PT, Daneshvar DH, Mez J, Solomon TM, Alosco ML, McKee AC. CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer's disease. PloS one. 2017:12(9):e0185541. doi: 10.1371/journal.pone.0185541. Epub 2017 Sep 26 [PubMed PMID: 28950005]
Alosco ML, Tripodis Y, Fritts NG, Heslegrave A, Baugh CM, Conneely S, Mariani M, Martin BM, Frank S, Mez J, Stein TD, Cantu RC, McKee AC, Shaw LM, Trojanowski JQ, Blennow K, Zetterberg H, Stern RA. Cerebrospinal fluid tau, Aβ, and sTREM2 in Former National Football League Players: Modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018 Sep:14(9):1159-1170. doi: 10.1016/j.jalz.2018.05.004. Epub 2018 Jul 23 [PubMed PMID: 30049650]
Alosco ML, Su Y, Stein TD, Protas H, Cherry JD, Adler CH, Balcer LJ, Bernick C, Pulukuri SV, Abdolmohammadi B, Coleman MJ, Palmisano JN, Tripodis Y, Mez J, Rabinovici GD, Marek KL, Beach TG, Johnson KA, Huber BR, Koerte I, Lin AP, Bouix S, Cummings JL, Shenton ME, Reiman EM, McKee AC, Stern RA, DIAGNOSE C. T. E. Research Project. Associations between near end-of-life flortaucipir PET and postmortem CTE-related tau neuropathology in six former American football players. European journal of nuclear medicine and molecular imaging. 2023 Jan:50(2):435-452. doi: 10.1007/s00259-022-05963-x. Epub 2022 Sep 24 [PubMed PMID: 36152064]
Asken BM, Rabinovici GD. Identifying degenerative effects of repetitive head trauma with neuroimaging: a clinically-oriented review. Acta neuropathologica communications. 2021 May 22:9(1):96. doi: 10.1186/s40478-021-01197-4. Epub 2021 May 22 [PubMed PMID: 34022959]
Su Y, Protas H, Luo J, Chen K, Alosco ML, Adler CH, Balcer LJ, Bernick C, Au R, Banks SJ, Barr WB, Coleman MJ, Dodick DW, Katz DI, Marek KL, McClean MD, McKee AC, Mez J, Daneshvar DH, Palmisano JN, Peskind ER, Turner RW 2nd, Wethe JV, Rabinovici G, Johnson K, Tripodis Y, Cummings JL, Shenton ME, Stern RA, Reiman EM, DIAGNOSE CTE Research Project Investigators. Flortaucipir tau PET findings from former professional and college American football players in the DIAGNOSE CTE research project. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2024 Mar:20(3):1827-1838. doi: 10.1002/alz.13602. Epub 2023 Dec 22 [PubMed PMID: 38134231]
Shahim P, Gill JM, Blennow K, Zetterberg H. Fluid Biomarkers for Chronic Traumatic Encephalopathy. Seminars in neurology. 2020 Aug:40(4):411-419. doi: 10.1055/s-0040-1715095. Epub 2020 Aug 2 [PubMed PMID: 32740901]
Ge X, Guo M, Li M, Zhang S, Qiang J, Zhu L, Cheng L, Li W, Wang Y, Yu J, Yin Z, Chen F, Tong W, Lei P. Potential blood biomarkers for chronic traumatic encephalopathy: The multi-omics landscape of an observational cohort. Frontiers in aging neuroscience. 2022:14():1052765. doi: 10.3389/fnagi.2022.1052765. Epub 2022 Nov 7 [PubMed PMID: 36420308]
Level 2 (mid-level) evidenceShahim P, Zetterberg H, Simrén J, Ashton NJ, Norato G, Schöll M, Tegner Y, Diaz-Arrastia R, Blennow K. Association of Plasma Biomarker Levels With Their CSF Concentration and the Number and Severity of Concussions in Professional Athletes. Neurology. 2022 Jul 26:99(4):e347-e354. doi: 10.1212/WNL.0000000000200615. Epub 2022 Jun 2 [PubMed PMID: 35654597]
Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R, Crowther RA, Newell KL, Ghetti B, Goedert M, Scheres SHW. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019 Apr:568(7752):420-423. doi: 10.1038/s41586-019-1026-5. Epub 2019 Mar 20 [PubMed PMID: 30894745]
Sáinz Pelayo MDP, Pelayo Vergara R, Albu S, Figueira C. [Experience with 4 clinical cases. Traumatic encephalopathy may be associated with a single traumatic brain injury?]. Rehabilitacion. 2022 Oct-Dec:56(4):383-387. doi: 10.1016/j.rh.2021.04.004. Epub 2021 Sep 17 [PubMed PMID: 34538654]
Level 3 (low-level) evidencePeacock WF, Kuehl D, Bazarian J, Singer AJ, Cannon C, Rafique Z, d'Etienne JP, Welch R, Clark C, Diaz-Arrastia R. Defining Acute Traumatic Encephalopathy: Methods of the "HEAD Injury Serum Markers and Multi-Modalities for Assessing Response to Trauma" (HeadSMART II) Study. Frontiers in neurology. 2021:12():733712. doi: 10.3389/fneur.2021.733712. Epub 2021 Dec 8 [PubMed PMID: 34956041]
Asken BM, Tanner JA, VandeVrede L, Casaletto KB, Staffaroni AM, Mundada N, Fonseca C, Iaccarino L, La Joie R, Tsuei T, Mladinov M, Grant H, Shankar R, Wang KKW, Xu H, Cobigo Y, Rosen H, Gardner RC, Perry DC, Miller BL, Spina S, Seeley WW, Kramer JH, Grinberg LT, Rabinovici GD. Multi-Modal Biomarkers of Repetitive Head Impacts and Traumatic Encephalopathy Syndrome: A Clinicopathological Case Series. Journal of neurotrauma. 2022 Sep:39(17-18):1195-1213. doi: 10.1089/neu.2022.0060. Epub 2022 Jun 6 [PubMed PMID: 35481808]
Level 2 (mid-level) evidencevan Amerongen S, Caton DK, Ossenkoppele R, Barkhof F, Pouwels PJW, Teunissen CE, Rozemuller AJM, Hoozemans JJM, Pijnenburg YAL, Scheltens P, Vijverberg EGB. Rationale and design of the "NEurodegeneration: Traumatic brain injury as Origin of the Neuropathology (NEwTON)" study: a prospective cohort study of individuals at risk for chronic traumatic encephalopathy. Alzheimer's research & therapy. 2022 Sep 1:14(1):119. doi: 10.1186/s13195-022-01059-8. Epub 2022 Sep 1 [PubMed PMID: 36050790]
Alosco ML, Mariani ML, Adler CH, Balcer LJ, Bernick C, Au R, Banks SJ, Barr WB, Bouix S, Cantu RC, Coleman MJ, Dodick DW, Farrer LA, Geda YE, Katz DI, Koerte IK, Kowall NW, Lin AP, Marcus DS, Marek KL, McClean MD, McKee AC, Mez J, Palmisano JN, Peskind ER, Tripodis Y, Turner RW 2nd, Wethe JV, Cummings JL, Reiman EM, Shenton ME, Stern RA, DIAGNOSE CTE Research Project Investigators. Developing methods to detect and diagnose chronic traumatic encephalopathy during life: rationale, design, and methodology for the DIAGNOSE CTE Research Project. Alzheimer's research & therapy. 2021 Aug 12:13(1):136. doi: 10.1186/s13195-021-00872-x. Epub 2021 Aug 12 [PubMed PMID: 34384490]
Adams JW, Alvarez VE, Mez J, Huber BR, Tripodis Y, Xia W, Meng G, Kubilus CA, Cormier K, Kiernan PT, Daneshvar DH, Chua AS, Svirsky S, Nicks R, Abdolmohammadi B, Evers L, Solomon TM, Cherry JD, Aytan N, Mahar I, Devine S, Auerbach S, Alosco ML, Nowinski CJ, Kowall NW, Goldstein LE, Dwyer B, Katz DI, Cantu RC, Stern RA, Au R, McKee AC, Stein TD. Lewy Body Pathology and Chronic Traumatic Encephalopathy Associated With Contact Sports. Journal of neuropathology and experimental neurology. 2018 Sep 1:77(9):757-768. doi: 10.1093/jnen/nly065. Epub [PubMed PMID: 30053297]